2023 年 48 巻 8 号 p. 469-479
2023 年 48 巻 8 号 p. 469-479
The use of doxorubicin (DOX) may contribute to cardiotoxicity, limiting its clinical application. Thiolutin (THL) has been found to exert protective roles in various biological activities, while its effects on DOX-induced cardiotoxicity are still uncovered. Cell counting kit 8 assay was utilized to detect cell viability and half maximal inhibitory concentration of THL in H9c2 cardiomyocytes. The level of lactate dehydrogenase (LDH), adenosine triphosphate (ATP), interleukin (IL)-18 and IL-1 beta (IL-1β) were measured using the corresponding detection kits, and flow cytometry determined cell apoptosis rate. The reactive oxygen species (ROS) accumulation was evaluated by utilizing immunofluorescence or flow cytometry assay. The protein levels of NLR family Pyrin domain 3 (NLRP3), pro-Caspase1, cleaved-Caspase1, gasdermin D (GSDMD) and cleaved-GSDMD (GSDMD-N) in H9c2 cells were detected by immunoblotting assay. The treatment of THL reduced H9c2 cell viability in a gradient-dependent manner. THL treatment reversed the DOX-induced inhibition of proliferation, decrease of ATP, up-regulation of LDH, IL-18, IL-1β and production of ROS, activation of NLRP3 and inflammasome-mediated pyroptosis in H9c2 cells. Additionally, NLRP3 knockdown abolished the effects of THL in DOX-treated H9c2 cells remarkably. This investigation proved that THL notably ameliorated DOX-induced apoptosis, oxidative stress, and pyroptosis in H9c2 cardiomyocytes. Besides, THL effectively inactivated DOX-induced NLRP3 inflammasome in H9c2 cells. These findings revealed a promising drug to assist DOX in its anti-cancer effects and protect the heart of patients.
Doxorubicin (DOX), the first anthracycline discovered in the 1960s (Cagel et al., 2017), is effective against the tumorigenesis of various cancers, including breast cancer (Cao et al., 2019), ovarian cancer (Mistarz et al., 2021) as well as bladder cancer (Khandelwal et al., 2018). Nevertheless, it is reported that DOX exerts non-negligible side effects, especially its significant cardiotoxicity (Zhang et al., 2020; Liang et al., 2019), which is likely to contribute to irreversible heart disease and ultimately congestive heart failure (Yang et al., 2013; Ma et al., 2013; Guo et al., 2018). Hence, the clinical application of DOX is greatly limited (Renu et al., 2018). Previous reporters have revealed that multiple biological processes, including oxidative stress, apoptosis and autophagy, are involved in DOX-related cytotoxicity (Songbo et al., 2019; Kobayashi et al., 2010; Gu et al., 2018). Additionally, there is evidence that DOX induces pyroptosis in cardiomyocytes (Tavakoli Dargani and Singla, 2019; Singla et al., 2019). Increasing numbers of studies have proved pyroptosis inhibition as a potential target for improving cardiovascular injury (Christidi and Brunham, 2021; Gu et al., 2021).
Pyroptosis is identified as a novel type of programmed cell death, also known as secondary necrosis (Silva, 2010). It is characterized by cell expansion and plasma membrane rupture, resulting in the outflow of cytokines and cytochylema, and the activation of inflammation-related responses (Jesenberger et al., 2000; Cervantes et al., 2008). Pyroptosis was initially found in infectious diseases (Friedlander, 1986), while it has since been discovered in the nervous and cardiovascular systems (McKenzie et al., 2020; Shi et al., 2021a). It is well known that pyroptosis can be activated by the typical inflammasome pathway, in which caspase-1, upon activation, cleaves the cytosolic gasdermin D (GSDMD) and forms a transmembrane pore through its N-terminus, thereby causing pyroptosis (Sun et al., 2019; Wang et al., 2017). In addition, NLR family Pyrin domain 3 (NLRP3) is capable of activating caspase-1, and the activated NLRP3 accelerates the activity transformation of interleukin-(IL-)1β, IL-18 as well as GSDMD (Kang et al., 2018). Emerging evidence also indicated that NLRP3 inflammasome is closely related to cardiovascular diseases, including hypertension, atherosclerosis, and heart failure (Tong et al., 2020). Therefore, inhibition of NLRP3 inflammasome has the potential to prevent the occurrence of pyroptosis and cardiovascular disease.
Thiolutin (THL), a zinc chelator, has been identified as compounds containing bicyclic dithiols and was first purified from Streptomyces luteum (Fig. 1A) (Kebaara et al., 2006; Celmer and Solomons, 1955). It is found that THL affects the activity of diverse malignancies including esophageal squamous cell carcinoma (Jing et al., 2021). Moreover, Ren et al. reported that THL inhibits NLRP3 inflammasome assembly, including NLRP3 binding to apoptosis-associated speck-like protein (ASC) and ASC spot formation (Ren et al., 2021). This finding suggested that THL may serve as a potential drug to prevent or treat diseases associated with abnormal NLRP3 inflammasome activation.

THL effectively attenuates the doxorubicin-induced injury in H9c2 cardiomyocytes. (A) The molecular structure of THL. (B) CCK-8 assay was employed to measure the toxicity of THL to H9c2 cardiomyocytes (*p < 0.05, **p < 0.01, ***p < 0.001). (C) The IC50 of THL in H9c2 cells detected via CCK-8 assay. (D) Cell viability detected via CCK-8 (***p < 0.001, ###p < 0.001). (E) The LDH release and (F) ATP contend in H9c2 cells (**p < 0.001, ***p < 0.001, ###p < 0.001). (G) Cell apoptosis detected via flow cytometry (***p < 0.001, ###p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001 versus control group. ###p < 0.001 versus DOX group.
The study aimed to explore the ameliorative effect and potential mechanism of THL on DOX-induced cardiomyocyte injury, indicating a new therapeutic strategy to reduce the occurrence of cardiotoxicity in patients.
The rat cardiomyocytes (H9c2) were brought from Procell Life Science & Technology Co., Ltd. (CL-0089, Wuhan, China) and were maintained in DMEM (L110KJ, BasalMedia, Shanghai, China) with 10% fetal bovine serum (FBS, FBS500, BasalMedia, Shanghai, China) and 1% penicillin-streptomycin (P/S, S110JV, BasalMedia, Shanghai, China). Besides, all cells were placed in a 37°C atmosphere with 5% CO2.
Cell treatmentH9c2 cells were treated with THL (HY-N6712, MCE, New Jersey, USA) at specific concentrations (0, 1, 10, 20, 40, 80, 160 or 320 nM) and 1 μM DOX (HY-15142A, MCE, New Jersey, USA) for 24 hr.
CCK-8 assayThe half-maximal inhibitory concentration (IC50) was evaluated by employing CCK-8 assay. In brief, H9c2 cardiomyocytes (3 × 103 cells/well) were cultured in 96-well plates. Subsequently, the cells were administered the indicated concentrations of THL (0, 1, 10, 20, 40, 80, 160 or 320 nM) for 24 hr. Then, 10 μL CCK-8 reagent (GK10001, GlpBio, CA, USA) were incubated with cells for another 2 hr, followed by measuring the absorbance at 570 nm. Additionally, cell viability was measured after treatment with THL at specific concentrations and 1 μM DOX.
Cell transfectionSmall interfering RNAs (siRNAs) targeting NLRP3 or control sequences were purchased from Kingsray Biotechnology Co., Ltd and transfected in H9c2 cardiomyocytes using Transfection Reagent I (790310P, Avanti, Alabama, USA), according to the manufacturer's protocol.
Detection of LDH and ATPH9c2 cardiomyocytes that underwent the abovementioned treatments were harvested. The level of LDH and ATP in cells was determined by a lactate dehydrogenase (LDH) detection kit (ml095304, Shanghai Enzyme Linked Technology Biology Co., Ltd., Shanghai, China) or ATP Assay Kit (S0026, Beyotime, Shanghai, China), according to the kit's instruction, respectively. The content of IL-1β or IL-18 in cell supernatant was measured with Rat IL-1β ELISA Kit (ab255730, Abcam, Cambridge, UK) or Rat IL-18 ELISA kit (KRC2341, Invitrogen, CA, USA) according to the kit's instruction, respectively.
Cell apoptosis analysisAn Annexin V-EGFP/PI double staining cell apoptosis detection Kit (KGA101, KeyGEN, BioTECH, Jiangsu, China) was introduced to detect cell apoptosis abiding by the kit's protocol. Briefly, H9c2 cardiomyocytes that underwent the abovementioned treatments were collected and stained by employing Annexin V-FITC and PI. Then apoptotic cells were recorded utilizing a FACScan flow cytometer (BD Biosciences, CA, USA).
Quantitative real-time PCR assayThe total RNA of H9c2 cells was isolated by utilizing TRIzol reagent (KGA1201, KeyGEN, BioTECH, Jiangsu, China) and reverse transcribed with cDNA First Strand Synthesis Kit (KGA1311, KeyGEN, BioTECH, Jiangsu, China). Then qRT-PCR analysis was performed using Quantitative PCR Premixed solution (MQ00801S, Monad, Wuhan, China) following the instruction of the manufacturer. GAPDH was considered as the control gene in this assay and NLRP3 expression was quantified employing the 2-ΔΔCT method. The primers sequences are presented in Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| NLRP3 | AAAGGAAGTGGACTGCGAGA | TTCAAACGACTCCCTGGAAC |
| GAPDH | GAACGGGAAGCTCACTGG | GCCTGCTTCACCACCTTCT |
ROS level in H9c2 cardiomyocytes was evaluated by testing the transformation of cell permeability from 2',7'-dichlorofluorescein diacetic acid (DCFH-DA) to fluorescein dichlorofluorescein using immunofluorescence (IF) or flow cytometry. Briefly, H9c2 cardiomyocytes subjected to the abovementioned treatments were fixed with 4% Paraformaldehyde Fix Solution (P0099, Beyotime, Shanghai, China) for 30 min and permeabilized using Triton X-100 Solution (ST797, Beyotime, Shanghai, China) for another 15 min. Then, cells were treated with DCFH-DA for 30 min. Next, the fluorescence intensity was observed employing a confocal light microscopy system (STELLARIS 5 Cryo, Leica, Weztlar, Germany) and a FACScan flow cytometer (BD Biosciences, CA, USA).
Western blot analysisThe total protein of H9c2 cells was collected using RIPA lysis buffer (KGP703, KeyGEN, BioTECH, Jiangsu, China), isolated on a 10% SDS‐polyacrylamide gel, and then transferred onto polyvinylidene fluoride membranes (KGP114, KeyGEN, BioTECH, Jiangsu, China). After blocking with 5% BSA Blocking Buffer (AC17124, Acmec, Shanghai, China), the membranes were incubated with the appointed primary antibody as exhibited in Table 2 overnight at 4°C. Next, the membranes were treated with corresponding secondary antibody and were observed by employing ECL Western Blotting Substrate (PE0010, Acmec, Shanghai, China). Finally, the expression level of indicated proteins was calculated using imageJ software (NIH, Bethesda, USA).
| Antibody | Manufacturer | Cat. no. |
|---|---|---|
| NLRP3 | Bioss Inc. | bs-10021R |
| Pro/Cleave-Caspase 1 | ABclonal Technology | A19654 |
| GSDMD-FL/N | Biorbyt | orb1150254 |
| β-actin | Abcam | ab8226 |
| Ubiquitin | Abcam | ab134953 |
H9c2 cells treated with DOX combined with a series of concentrations of THL (0, 1, 10, 20, and 40 nM) were subjected to the administration of MG132 with a concentration of 10 μM for 6 hr. Then, cells were harvested, lysed, and immunoprecipitated by NLRP3 antibody. Finally, the precipitated proteins were exploited to detect the level of ubiquitination for NLRP3 using western blot.
Statistical analysisAll experiments were conducted 3 times. All data were analyzed employing GraphPad Prism 8.0 software (Graphpad, San Diego, USA). Two‐tailed Student's t test (in two groups) and ANOVA with Tukey's test (in multiple groups) were used to evaluate statistical significance. *p < 0.05, **p < 0.01, ***p < 0.001, ##p < 0.01 and ###p < 0.001 served as statistically significant.
H9c2 cardiomyocytes were treated with THL at different doses for 24 hr. Then the cell viability was measured to screen for concentrations that were not obviously toxic to H9c2 cells for subsequent experiments. The results illustrated that THL at concentrations of 1, 10, 20 and 40 nM exhibited minimal cytotoxicity, especially at 1, 10 and 20 nM. (Fig. 1B). Besides, the IC50 of THL in H9c2 cells was 94.15 nM (Fig. 1C). The detailed role of THL in cardiomyocyte injury induced via 1 μM DOX was determined using CCK-8 assay. As shown in Fig. 1D, the treatment of DOX dramatically reduced viability in H9c2 cells, which was effectively recovered by THL at concentrations of 10, 20, or 40 nM. In combination with the IC50 of THL in H9c2 cells, the concentration of 20 nM was selected for the following experiments. Given the important roles of LDH release in cardiomyocyte injury, we examined LDH levels in cell supernatant. The data demonstrated that DOX treatment increased LDH release, while 20 nM THL reversed these effects in H9c2 cells. Besides, LDH levels were not affected by THL treatment alone (Fig. 1E). Additionally, the ATP assay indicated that compared with the control group, THL exerted little role in ATP content, whereas DOX remarkably decreased ATP accumulation. However, THL co-treatment effectively reversed the DOX-induced decrease in ATP levels (Fig. 1F). Furthermore, H9c2 cell apoptosis was up-regulated in the DOX group, while THL treatment notably reversed DOX-induced cell apoptosis. As well, THL alone did not significantly change apoptosis in H9c2 cardiomyocytes (Fig. 1G). These findings indicated that THL effectively ameliorated DOX-induced cardiomyocyte injury in H9c2 cells.
THL decreases DOX-induced oxidative stress and inflammation in H9c2 cardiomyocytesTo investigate the role of THL in DOX-induced oxidative stress and inflammatory response, the cellular ROS production and the levels of inflammatory cytokines (IL-18 and IL-1β) were tested. As shown in Fig. 2A and 2B, the administration of DOX significantly increased ROS accumulation in H9c2 cells, which was reversed by THL treatment. Besides, ROS was not influenced in the THL group compared to the control group (Fig. 2A and 2B). Subsequently, the release of IL-18 and IL-1β was up-regulated by DOX treatment remarkably, while THL co-treatment effectively reduced this effect in cell supernatant. Similarly, the effects of THL alone on IL-18 and IL-1β levels were not significantly different (Fig. 2C and 2D). These results suggested that THL obviously relieved oxidative and inflammation induced via DOX in H9c2 cardiomyocytes.
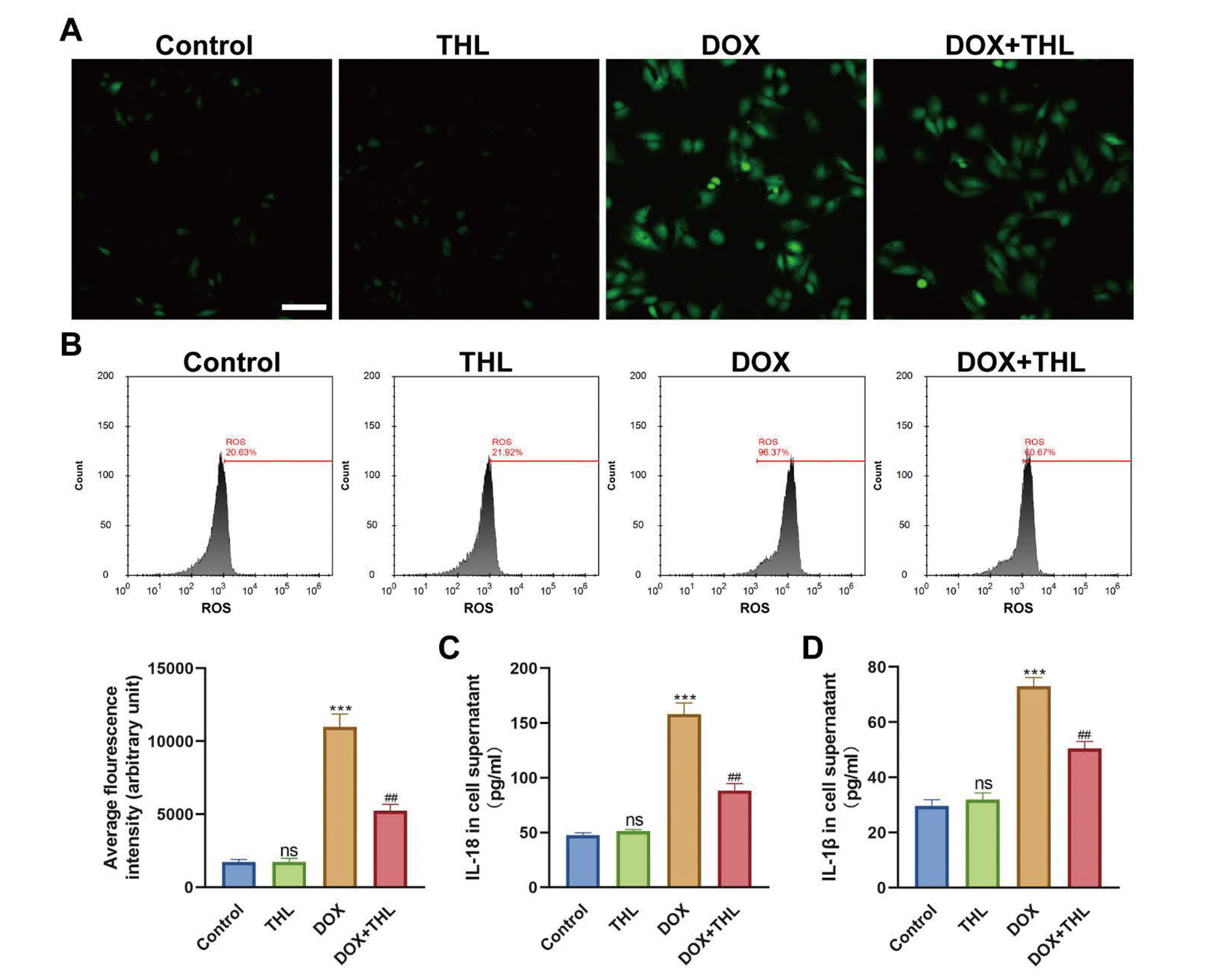
THL decreases DOX-induced oxidative stress and inflammation in H9c2 cardiomyocytes. (A) ROS accumulation detected via IF (Scale bar: 100 μm). (B) ROS accumulation detected and quantified via flow cytometry (***p < 0.001, ##p < 0.01). The expression level of IL-18 (C) and IL-1β (D) in cell supernatant (***p < 0.001, ##p < 0.01). ***p < 0.001 versus control group. ##p < 0.01 versus DOX group.
To further explore whether THL exerts its protective role in NLRP3-mediated pyroptosis in DOX-treated H9c2 cells, we measured the expression levels of NLRP3 and pyroptosis-related proteins, including pro-Caspase1, cleaved-Caspase1, GSDMD-N, and GSDMD-FL. The results illustrated that DOX administration notably increased the protein level of NLRP3, and the ratio of cleaved-Caspase1/pro-Caspase1 as well as the ratio of GSDMD-N/GSDMD-FL. However, the treatment of THL effectively reversed these effects in H9c2 cells. Besides, THL alone had no obvious role in the expression of these proteins (Fig. 3). These data demonstrated that THL notably alleviated DOX-induced pyroptosis related to NLRP3 inflammation in H9c2 cells.

THL suppresses the activation of NLRP3 inflammasome in DOX-induced H9c2 cardiomyocytes. (A) The protein level of NLRP3, pro-Caspase1, cleaved-Caspase1, GSDMD-N and GSDMD-FL detected via western blot analysis (***p < 0.001, ##p < 0.01). ***p < 0.001 versus control group. ##p < 0.01 versus DOX group.
Since a recent study demonstrated that thiolutin can suppress the expression of NLRP3 via regulating its ubiquitination process (Ren et al., 2021), the effect of thiolutin on the expression and ubiquitination of NLRP3 was explored. The results showed that the ubiquitination of NLRP3 in DOX-induced H9c2 cells was gradually increased with the increasing concentration of THL (Fig. 4A), suggesting that THL targets NLRP3 expression by regulating NLRP3 ubiquitination. Subsequently, we further confirmed whether the suppressive role of THL on NLRP3 inflammasome-associated pyroptosis in DOX-treated H9c2 cells is mediated via NLRP3. Firstly, the NLRP3 expression was inhibited by using siRNA technology (Fig. 4B). For the H9c2 cells transfected with si-NC, the results were similar to normal H9c2 cells, showing that THL effectively alleviated DOX-induced death, oxidative stress, and inflammation (Fig. 4C-I). In the context of NLRP3 depletion, DOX stimulation still caused cardiomyocyte injury in H9c2 cells, as revealed by decreased cell viability and ATP content, and increased LDH release (Fig. 4C-E). However, THL administration exerted no protective effect on DOX-treated H9c2 cells after NLRP3 knockdown (Fig. 4C-E). Moreover, in NLRP3-silenced H9c2 cells, there was no change in DOX-induced apoptosis, ROS production, and IL-18 and IL-1β release after THL treatment (Fig. 4F-I). Collectively, these investigations proved that THL exerts its protective roles in DOX-induced H9c2 cardiomyocyte injury at least partly by targeting NLRP3 expression.
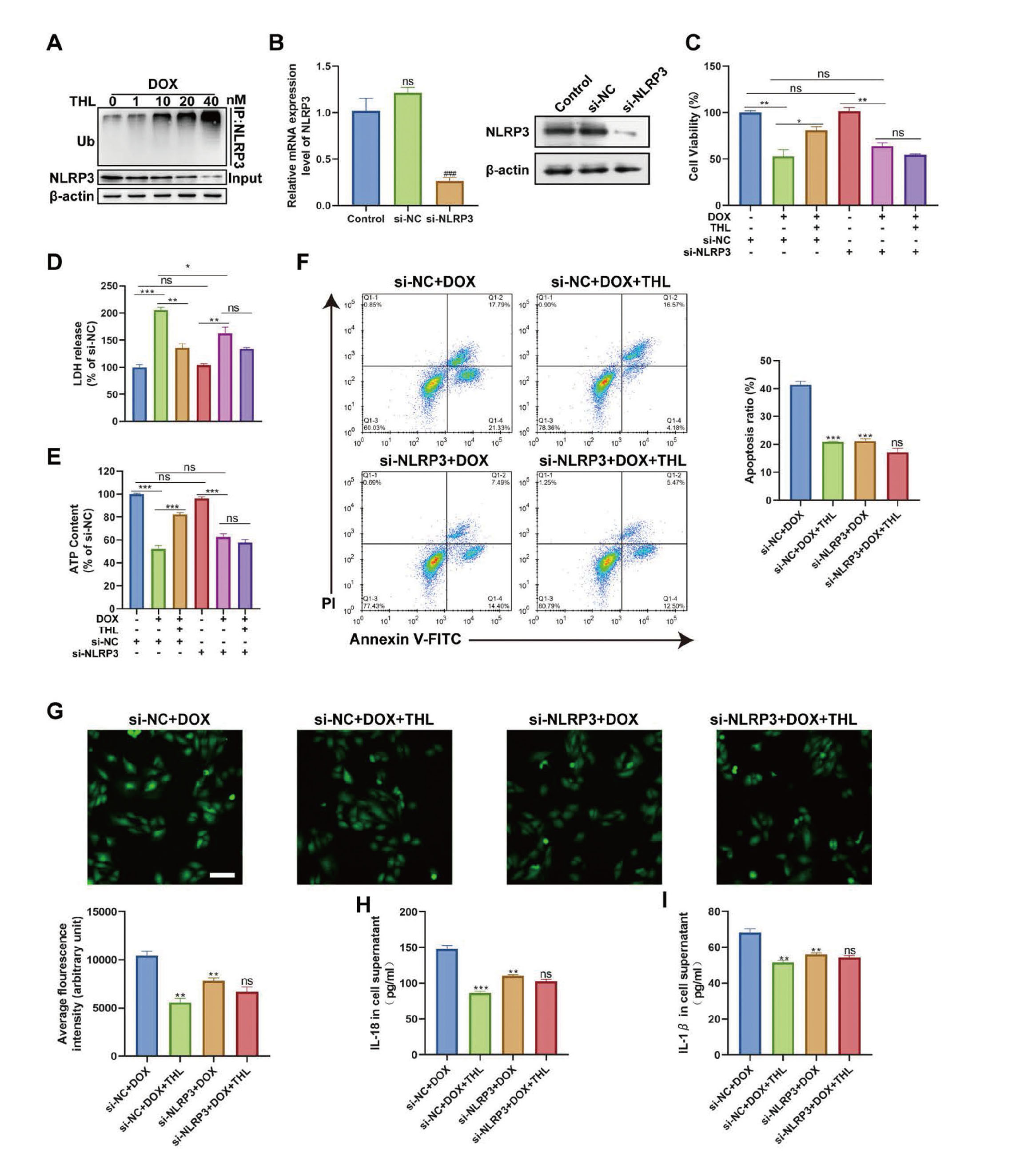
The effect of THL on the DOX-induced H9c2 cardiomyocytes in the context of NLRP3 depletion. (A) In vitro ubiquitination assay for DOX-induced H9c2 cells with a series of concentration of THL. (B) The expression level of NLRP3 at mRNA and protein level (###p < 0.001). (C) Cell viability was detected by CCK-8 (*p < 0.05, **p < 0.01, ns no significance). (D) The LDH release and (E) ATP contend in H9c2 cells (*p < 0.05, **p < 0.01, ***p < 0.001, ns no significance). (F) Cell apoptosis detected via flow cytometry. (G) ROS accumulation detected and quantified via flow cytometry. The expression level of IL-18 (H) and IL-1β (I) in cell supernatant. (F-I, **p < 0.01 and ***p < 0.001 versus si-NC + DOX group, ns no significance, versus si-NLRP3 + DOX group).
DOX, a widely used anti-cancer drug, has been severely limited in clinical application due to its cardiotoxicity (Fridrik et al., 2016). Although previous studies have elucidated part of the mechanism of DOX-induced cardiotoxicity, it remains controversial. Besides, increasing evidence pointed out that various pathological processes such as up-regulated ROS production and oxidative stress (Wenningmann et al., 2019), the release of pro-inflammatory markers (Shi et al., 2020), apoptosis (Shi et al., 2020), and pyroptosis (Christidi and Brunham, 2021) are involved in the development of DOX-induced cardiotoxicity. However, there exists currently no effective drug to alleviate the side effects induced via DOX. Therefore, it is crucial to reveal a reagent blocking the heart injury after DOX administration. THL, a dithiopyrrolidone compound, has a spectrum of antibacterial and antifungal activities (Seneca et al., 1952). Accumulating reports verified that antiangiogenic agents combined with chemotherapeutic agents (including DOX) exhibits better anti-cancer effect in a variety of tumors such as breast cancer (Shi et al., 2021b), osteosarcoma (Oshiro et al., 2021), and liver cancer (Niu et al., 2016). Emerging evidence has identified THL as a vital angiogenesis inhibitor (Kebaara et al., 2006; Minamiguchi et al., 2001). Nevertheless, whether THL cooperates with DOX in contributing to cell death and alleviating DOX-induced cardiomyocyte injury needs to be further investigated. In line with previous studies (Zhao et al., 2018; Zhang et al., 2020), our present investigation demonstrated that DOX administration resulted in myocardial injury by detecting the viability, apoptotic rate, and the levels of LDH and ATP in H9c2 cells. Interestingly, the co-treatment of THL effectively ameliorated DOX-induced injury in H9c2 cardiomyocytes.
It is reported that oxidative stress is a vital cause of DOX-induced cardiotoxicity. The use of DOX blocked the expression of genes related to mitochondrial biogenesis, damaged the structure and function, and disrupted the normal metabolic activities in mitochondria, thereby upregulating the ROS production (Zhang et al., 2012; Mukhopadhyay et al., 2010; Octavia et al., 2012). As expected, this work verified that DOX increased the level of ROS in H9c2 cells, which was obviously eliminated by the treatment of THL. These findings illustrated that THL alleviated DOX-induced oxidative stress in cardiomyocytes.
Previous evidence has revealed that the release of proinflammatory cytokines, such as IL-18 and IL-1β, is closely associated with DOX-induced cardiotoxicity (Zhang et al., 2021). Consistently, this study proved that DOX administration increased the level of IL-18 and IL-1β in cell supernatant, while the treatment of THL notably reduced that. Several studies reported that IL-1β is involved in promoting oxidative stress and ROS production (Yang et al., 2007; Liu et al., 2021). This may explain the effect of THL on the oxidative stress in DOX-induced cardiomyocytes. Additionally, pyroptosis, a recently proposed method of programmed cell death, is able to accelerate the production of inflammatory makers and facilitate the process of cardiomyocyte injury induced by DOX (Tavakoli Dargani and Singla, 2019). The classical pyroptosis pathway relies on caspase-1 activation. The NLRP3 inflammasome is activated in response to influenza virus, extracellular ATP, amyloid-β and other relevant signals, thereby activating caspase-1. Activated caspase-1, on the one hand, cleaves GSDMD to produce active GSDMD-N peptide, which causes pyroptosis by inducing cell rupture. In contrast, above caspase-1 cleaves the precursors of IL-18 and IL-1β to release activated IL-18 and IL-1β, which eventually leads to the aggregation of inflammatory cells (Rathinam and Chan, 2018; Franchi et al., 2009). Recently, increasing findings discovered that the suppression of NLRP3 inflammasome obviously decreased DOX-induced cardiac function injury (Marchetti et al., 2015). Moreover, THL inhibits the activation of NLRP3 inflammasome in murine bone marrow derived macrophages (Ren et al., 2021). However, whether THL exerts its protective effect in H9c2 cardiomyocytes treated with DOX by inactivating NLRP3 inflammasome remains unclear.
Subsequently, our investigation revealed that DOX up-regulated the expression of NLRP3 and proteins related to pyroptosis in H9c2 cells, which was effectively reversed through the administration of THL. These findings verified that THL suppressed NLRP3 inflammasome-associated pyroptosis induced via DOX treatment in cardiomyocytes. At the level of molecular mechanism, our data showed that the protective effects of THL on viability, apoptosis, oxidative and inflammatory response in DOX-treated H9c2 cells were not observed in the context of NLRP3 depletion, which implied that THL protected cardiomyocytes from DOX-induced injury possibly by regulating NLRP3-mediated NLRP3 inflammasome.
This study was the first to uncover the protective role of THL in DOX-induced injury in cardiomyocytes and link these effects to the activation of NLRP3 inflammasome. However, there are some limitations in this study. For example, the detailed mechanism of THL regulating NLRP3-mediated pyroptosis will be further explored. In addition, more experiments are needed to determine whether THL exerts cardioprotective effects in the presence of DOX in vivo.
In summary, this work revealed that THL effectively alleviated DOX-induced cell injury, oxidative stress, and inflammatory response in H9c2 cardiomyocytes at least partly by inhibiting NLRP3 inflammasome-mediated pyroptosis, which provides a promising agent to alleviate cytotoxicity induced via DOX.
Conflict of interestThe authors declare that there is no conflict of interest.
FundingThis work was supported by grants from National Natural Science Foundation of China (81873486), the Science and Technology Development Program of Jiangsu Province-Clinical Frontier Technology (BE2022754).