2023 年 48 巻 8 号 p. 481-486
2023 年 48 巻 8 号 p. 481-486
Lead (Pb) exposure induces testicular damage and infertility. The aim of this study was to analyze and compare the therapeutic effects of antioxidants or vitamin D and calcium, which have previously been shown to reduce the toxic effects of Pb co-exposure, in rats. Rats were exposed to Pb for 28 days and subsequently treated with antioxidant (melatonin, silymarin), vitamin D and calcium (VitDCa) or a combination (melatonin or silymarin with VitDCa) for 28 days. Control groups included untreated rats (no Pb exposure or therapy), rats exposed only to melatonin or silymarin and rats exposed to Pb without post exposure therapy. Pb exposure induced testicular damage, increased blood lead level (BLL) and reduced serum testosterone level (STL). Rats exposed to Pb and left untreated for 28 days showed persistent pathological testicular alterations. The two treatments that were most effective in reversing pathological testis damage and restoring spermatogenesis were melatonin and silymarin. However, silymarin and melatonin treatment resulted in significantly different serum testosterone levels in rats. Whereas melatonin therapy reduced serum testosterone to levels lower than those in control rats, silymarin increased serum testosterone to levels higher than those in controls. Our pathological analysis of testes revealed that melatonin promoted spermatogenesis and regression of Pb exposure-induced degenerative changes, despite the associated reduction in serum testosterone levels. This result suggests that circulating testosterone may not have an important role in spermatogenesis. Collectively, our results suggest that melatonin and silymarin are effective therapies against the toxic effects Pb exposure in the male reproductive system.
Lead (Pb) is a heavy metal with multiple applications, Lead exerts systemic toxic effects that include neurodevelopmental delay in children, behavioral alterations, nephrotoxicity, anemia, and hypertension. One study of 80 Pb-exposed battery factory workers (7-15 years exposure) showed an increase in sperm abnormalities and hormonal parameters (Naha et al., 2005). Furthermore, Pb exposure has been shown to reduce the steroidogenic activity of isolated Sertoli cells (Wiebe et al., 1983). Lead induces toxic effects through two main molecular mechanisms. The first is substitution of divalent ions, predominantly calcium and zinc, in protein binding sites (Dudev et al., 2018). The second is induction of oxidative stress (Ma et al., 2017). In light of these mechanisms, a number of studies have tested the protective role of co-administration of antioxidants or a mix of vitamin D (VitD) and calcium. Melatonin is a hormone secreted mainly in the pineal gland that reduces oxidative stress (Reiter et al., 2014). Silymarin, a polyphenolic compound extracted from the seeds of the milk thistle plant, is also an antioxidant (Taleb et al., 2018). Vitamin D (VitD) is a hormone involved in calcium homeostasis, expression of the VitD receptor (VDR) and VitD-metabolizing enzymes in the male reproductive system suggests that VitD is produced locally and exerts autocrine action. In animals, VitD produces beneficial effects on male fertility through modulation of hormone production (Zanatta et al., 2011). The aim of this study was to assess the therapeutic effects of melatonin, silymarin, VitD and Ca (VitDCa), as well as melatonin or silymarin combined with VitDCa, in Pb-exposed rats. To this end, we exposed rats to Pb and, following the indicated treatments, analyzed and compared testis pathology, testosterone serum level, and blood lead level (BLL) in treatment and control groups.
Lead acetate trihydrate, Pb (CH3CO2)2.3H2O purity 99.99% from Sigma 215902, Melatonin Sigma M5250 98% TLC, Silymarin Sigma S0292, VitD oral ampoules (100,000 IU/2 mL) from TRB, Calcium ampule powder 0.090 g ionic calcium SANDOZ, Lab Rat testosterone Elisa kit ABCAM ab285350.
Animals Ethical approvalThe procedures were carried out in accordance with the Official Mexican Standard: NOM-062-ZOO-1999 for lab animals use. This research was reviewed and authorized by the Ethics Committee of the Neurobiology Institute at the Universidad Nacional Autonoma de Mexico (UNAM), (approval number 146). Rats were supplied and taken care of by the vivarium of Neurobiology Institute Queretaro (NBI) UNAM.
Male Wistar rats raised in the vivarium (170-190 g body weight) 6 weeks age, were housed in groups of three in standard cages with free access to water and food (Rodent Lab Standard Diet), under a 12/12 hr light/dark cycle, RT 22-24°C. There were three rats per group, and the experiments were repeated in triplicate. Rat groups were orally administered lead acetate at a dose of 75 mg/kg/body weight (BW)/d. All experiments were performed in accordance with recommended international ethical standards and were handled following the guidelines of the Bioethics Committee of Experiments on Animals of the INB-UNAM. Experimental groups: C, control: untreated rats not exposed to Pb or therapeutics; Pb, exposed to Pb for 28 days and assessed immediately; M, melatonin (10 mg/kg/BW/d dose) according to previous work (Martínez-Alfaro et al., 2013) exposed for 28 days and assessed immediately; S silymarin (200 mg/kg/BW/d dose) according to previous work (Alcaraz-Contreras et al., 2016) exposed to silymarin for 28 days and assessed immediately. Treatment with VitD1200 IU/kg 3 days/week and calcium 1500 mg/kg 5 days/week;) for 4 weeks. According to the dose conversion equation between rat and human the total amount of VitD (232 IU/kg/BW/d) and Calcium 299 mg/kg/BW/d received from diet and therapeutic regimen were equal to 1.8 fold the daily requirement of VitD 800 IU/kg/BW/d and Calcium 1000 mg/kg/BW/d (Nair and Jacob, 2016) ; Pb 1NT, exposed to Pb for 28 days followed by 28 days without therapeutics; Pb 1TM, exposed to Pb for 28 days followed by 28 days of melatonin therapy; Pb 1TS, exposed to Pb for 28 days followed by 28 days of silymarin therapy; Pb 1TVitDCa, exposed to Pb for 28 days followed by 4 weeks as described before therapy; Pb 1TS+VitDCa, exposed to Pb for 28 days followed by 28 days of combined silymarin + VitDCa therapy; Pb 1TM+VitDCa, exposed to Pb for 28 days followed by 28 days of combined melatonin + VitDCa therapy (4 weeks as described before therapy). Melatonin administration was done between 7 and 8 pm and the rest of compounds in the morning between 8 and 9 am.
TestosteroneSerum testosterone level was determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions.
BLL measurementsWhole blood was first acid digested by adding 1 mL of concentrated nitric acid to a 1-mL aliquot of each sample, followed by heating to 110°C for 4 hr. After bringing the volume to 2.0 mL with deionized water, samples were analyzed using an Agilent 4100 MP-AES atomic emission spectrometer equipped with an SPS3 autosampler and employing a Mira Mist Teflon nebulizer and a single-pass glass cyclonic spray chamber controlled by Agilent MP Expert software (actualized from 4200 MP-AES). Unless stated otherwise, the instrument operating conditions were as follows: nebulizer pressure, 240 kPa; pump, 12 rpm; nitrogen pressure, 140 psi; plasma viewing position, -10 for Pb and 10 for Ca; stabilization time, 20 sec; integration time, 3 sec; wavelengths, 283.305 nm (Pb) and 396.847 nm (Ca). Multi-element standard solutions were prepared in 2% v/v nitric acid, which was also used as a blank. Six-point calibration curves covered the concentration ranges 0.05–5.0 mg/L for Ca and 0.5–10 mg/L for Pb. Auto background correction was applied, and the analytical signal was acquired as peak height for each element. Prior to their introduction, MP-AES–hydrolyzed samples were microcentrifuged (13,000 × g, 10 min) and then diluted with deionized water as follows: 1:30 for Ca and 1:5 for Pb.
Statistical AnalysisDifferences between all groups were compared using parametric one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test for testosterone measurements and for BLL analysis. In both cases, differences with P-values < 0.05 were considered statistically significant. Graph Pad Prism, version 7, was used for statistical analyses and graphing.
Histopathological AnalysisImmediately after removal, each testis was fixed in 10% formalin for 5 days. After dehydration and clearing (1 hr each), tissue was embedded in paraffin and cut into 5-μm thick sections. Sections were then stained with hematoxylin and eosin and analyzed histopathologically under a light microscope.
Histology of the testes Fig. 1

Histology of the testes, magnification 100x. A, Control. B, Pb. C, Pb 1NT. D, Pb 1TM. E, Pb 1TS. F, Pb 1TVDCa. G, Pb 1TS+VDCa. H, Pb 1TM+VDCa. Magnification 1000x I, Pb. J, Pb 1NT.
C All stages of spermatogenesis were present in control rats (from primary spermatocytes to spermatozoa). Sertoli cells were situated directly over the basement membrane.
Pb Seminiferous tubules of rats recently exposed to Pb showed degenerative changes, including thickening of the basement membrane, a decrease in germ cell numbers, a reduction in spermatid differentiation and a severe reduction in sperm production. Cells exhibited a vacuolated cytoplasm (arrow Fig. 1, B and Fig. 1, I) with pyknotic nuclei (star Fig. 1, I).
Pb 1NT Rats exposed to Pb for 1 month followed by a treatment-free month showed persistent degenerative changes. These included thickening of the basement membrane, detachment of spermatogonia cells with pyknotic nuclei (star Fig. 1, J close up), vacuolated cytoplasm (arrow Fig. 1, J close up) and lipofuscin accumulation (rectangle Fig. 1, J close up). A severe loss of germ cells with minor sperm formation was still noted.
Pb 1TS Testes of rats exposed to Pb for 1 month and subsequently treated with silymarin were very similar to control testes, with all stages of spermatogenesis present. Spermatogenic cells layers were preserved, and lumen sperm counts were high.
Pb 1TM Testes of rats exposed to Pb for 1 month and then treated with melatonin exhibited regression of degenerative changes induced by Pb exposure. The appearance of spermatids and stages of spermatogenesis were normal, and sperm production was restored.
Pb 1VDCa Testicular biopsies of rats exposed to Pb for 1 month and later treated with VitDCa (Fig. 1, F) revealed partial recovery showing a reduction in spermatogenesis (reduction in the number of spermatocytes, round and elongated spermatids and reduced sperm in comparison to control testes (Fig. 1, A).
Pb 1TM+VDCa Testes of rats exposed to Pb for 1 month and subsequently treated with a combination of melatonin and VitDCa showed partial recovery of Pb exposure effects.
Pb 1TS+VDCa Although all stages of spermatogenesis were present in testicular sections, they showed little regression of Pb-induced alterations, cell vacuolation, or hypospermatogenesis with low sperm production.
M and S Testicular biopsies of rats exposed to melatonin or silymarin were similar to control rats; no figures were included.
Blood lead levelsOnly two samples of M and S conditions were evaluated, and these did not show differences (2.6 and 2.2 μg/dL mean respectively) with control values. This means that Pb contamination was not found in M and S.
In Fig. 2, all groups exposed to Pb for 28 days and successively underwent therapeutical treatments after Pb exposure* reduce the BLL in comparison to the group exposed to Pb for 28 days and subsequently left untreated for 28 days (Pb 1NT) P < 0.05. However, there is no difference between the BLL in all groups therapeutically treated after Pb exposure. All therapeutics reduce BLL at similar level.

Blood Lead Levels. Effect of Pb exposure and subsequent treatments on BLL (μg/dL) in rats. BLL increases in all experimental groups, that include Pb exposure throughout 28 days (denoted by #), compared to the control group C (P < 0.0001). All therapies applied after Pb exposure (denoted by *) reduce the BLL in comparison to BLL found in group (Pb 1NT), without post exposure therapy (P < 0.05). All groups exposed to Pb for 28 days and successively underwent therapeutical treatments after Pb exposure, reduce the BLL in comparison to the group exposed to Pb for 28 days and subsequently left untreated for 28 days (Pb 1NT) P < 0.05. Statistical Analysis one-way ANOVA (P < 0.05), Tukey’s multiple comparisons test).
Considering that BLL of all groups analyzed (except control) were toxic at the end of study (56 days in total). It is probable that all stages of spermatogenesis were affected since the duration of cycle spermatogenesis is at least 54 days in rats.
The Pb dose of 75 mg was used to have BLL < 20 μg/dL since there is not sufficient evidence that BLL below this level induces sperm alteration (Kumar, 2018) Therapeutic administration of antioxidants (silymarin or melatonin) or VitDCa reduced BLL in rats exposed to Pb for 28 days compared with rats without therapy 28 days following the end of Pb exposure.
Serum testosterone levelsIn Fig. 3, Pb exposure reduces STL (Pb group). Two groups (Pb 1TVDCa* and Pb 1TS*) do not show significant difference with the control group (C) These two therapies restored STL to control values, Pb1TS and Pb1TVDCa. The other therapies, Pb 1TM, Pb1TMVDCa or Pb1TSVDCa, have STL below control values. Two treatments induced opposite changes in serum testosterone levels, with melatonin (M and Pb1TM) reducing and silymarin (S) increasing levels compared with controls. For Pb1TS a high mean was found but a high dispersion reduces the statistical significance.
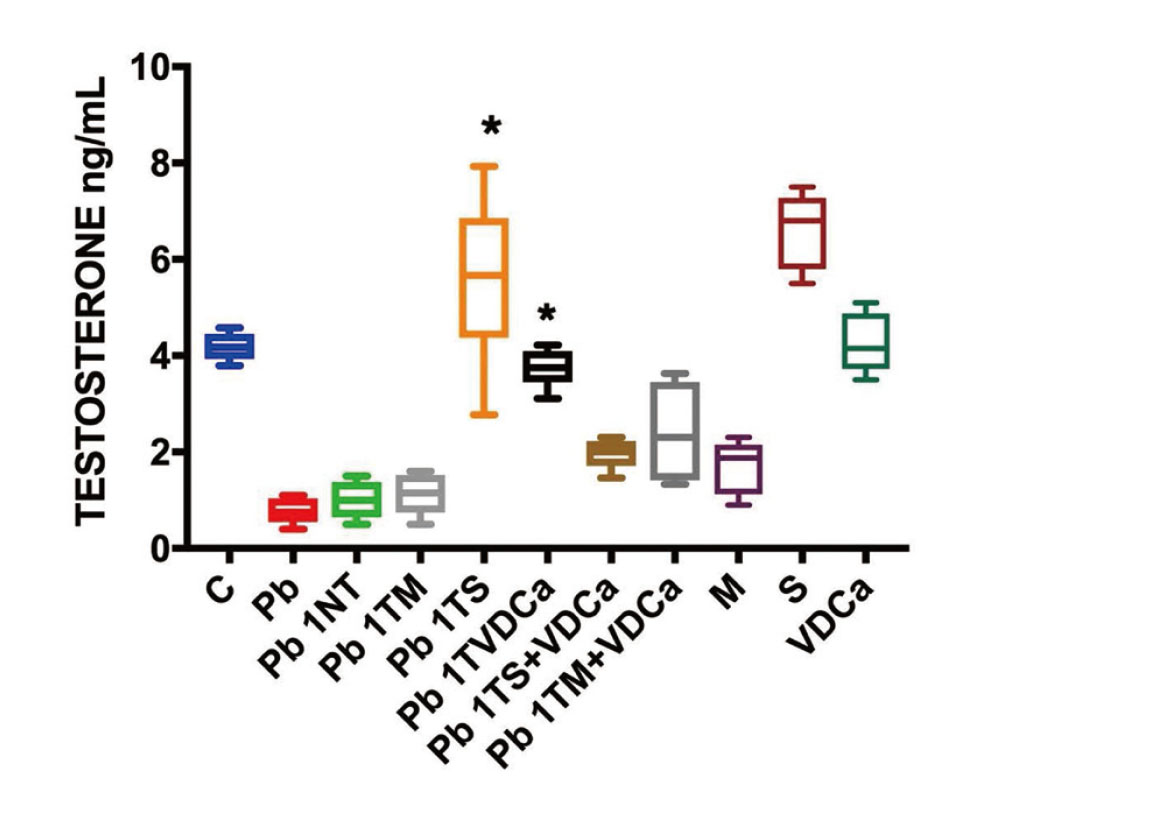
Serum Testosterone Level. Effects of Pb exposure and subsequent therapies on serum testosterone levels in rats. Two groups (Pb 1TVDCa* and Pb 1TS*) do not show significant difference with the control group (C). All other experimental conditions (8 groups) show a mean STL difference with the control group. Statistical Analysis one-way ANOVA (P < 0.05), Tukey’s multiple comparisons test).
There are two mechanisms proposed to reduce serum testosterone level for melatonin. In rats, melatonin decreases the testicular activity of two steroidogenic enzymes, 3β-HSD and 17β-HSD (Maitra and Ray, 2000). In a second study, melatonin receptors (Mel1a) were identified in Leydig cells isolated from Syrian hamsters, and melatonin was found to downregulate the expression of genes encoding key steroidogenic enzymes (Frungieri et al., 2005).
The effects of melatonin on spermatogenesis observed in our study could reflect the action of estrogen, as suggested by Carreau et al., who proposed that E2 (estrogen) and not testosterone is a regulator of spermatogenesis (Carreau et al., 2008). Consistent with this idea, it has been reported that melatonin administration alone induces an increase in E2 plasma concentration and sperm count in rats. This melatonin effect on estrogen could counter the reduction in serum estrogen observed in rats exposed to Pb. One proposed mechanism of Pb gonadotoxicity is a reduction in serum estrogen and testosterone. E2 is synthesized from androgen by the P450 enzyme, and expression of P450 (Cyp19) mRNA in testes is reduced by Pb (El-Magd et al., 2017).
Similar to melatonin, VitD treatment increases semen quality (sperm motility, count and fertility), despite having no effect on circulating levels of testosterone (Ramlau-Hansen et al., 2011). One in vitro study on cultures of Leydig cell shows that VitD treatment induced mRNA expression of enzymes involved un testosterone synthesis, increasing testosterone secretion in testes (Hofer et al., 2014). Testosterone levels in rats treated with VitD and Ca were similar to those in control rats in our study. Probably, calcium administration changes the result.
As noted above, silymarin increased testosterone serum levels in rats previously exposed to Pb, raising it to level higher than that in controls. This result is in agreement with a previous report showing that administration of silymarin in mice increases serum testosterone levels (Faraji et al., 2019).
Current controversies regarding increases in spermatogenesis with melatonin administration or semen quality with VitD administration in relation to low levels of circulating testosterone levels underscore questions regarding the role of circulating testosterone in the regulation of spermatogenesis.
Our pathological analysis of testes showed that individually applied melatonin and silymarin are the better therapeutic options for reducing the toxic effects of Pb exposure among those treatments evaluated. By comparison, we found that, in our experimental conditions, testis from rat treated with a mix of VitD and Ca showed partial regression of Pb damage.
Testosterone is the major androgen hormone in males, and testes are the source of more than 95% of the circulating hormone. Our results showed no relationship between serum testosterone levels and the pathological state of the testes.
The results reported herein should be considered in light of some limitations, the most notable of which is that intratesticular testosterone and estrogen levels were not measured.
To our knowledge, this is the first study to compare the therapeutic efficacy of melatonin, silymarin and vitamin D and calcium after Pb exposure in rats.
This work was supported by Universidad de Guanajuato DAIP CIIC (grant number 046/2022 and 108/2022).
MVZ José Martín García Servín, Dra. María A Carbajo Mata and Dra. Alejandra Castilla León for supply and care of laboratory animals in the vivarium of Neurobiology Institute Queretaro (NBI) UNAM.
Conflict of interestThe authors declare that there is no conflict of interest.