2024 年 49 巻 1 号 p. 9-26
2024 年 49 巻 1 号 p. 9-26
Amphetamine-type stimulants are abused worldwide, and methamphetamine (METH) accounts for a large majority of seized abused drug cases. Recently, the paternal origin of health and disease theory has been proposed as a concept wherein paternal factors influence descendants. Although METH abuse is more common among males, its effects on their descendants were not examined. Therefore, we investigated the effects of paternal METH exposure on F1 and F2 levels in a mouse model. Sires were administered METH for 21 days and mated with female mice to obtain F1 mice. Growth evaluations (number of births, survival rate, body weight, righting reflex, cliff avoidance tests, and wire-hanging maneuver) were performed on F1 mice. Upon reaching six weeks of age, the mice were subjected to spontaneous locomotion, elevated plus-maze, acute METH treatment, and passive avoidance tests. Additionally, RNA-seq was performed on the striatum of male mice. Male F1 mice were mated with female mice to obtain F2 mice. They were subjected to the same tests as the F1 mice. Paternal METH exposure resulted in delayed growth and decreased memory function in F1 mice, overweight in F2 mice, decreased METH sensitivity, and reduced anxiety-related behaviors in female F2 mice. Enrichment analysis revealed significant enrichment of terms related to behavior in F1 and protein folding in F2. These results indicated that the effects of paternal METH exposure vary across generations. The effects of paternal factors need to be examined not only in F1, but also in F2 and beyond.
Amphetamine-type stimulants (ATS) are widely abused worldwide, and the number of ATS seized in 2021 is the highest ever (UNODC Research, 2023). Methamphetamine (METH) accounts for the majority of ATS and the METH market continues to expand, particularly in Southeast Asia (UNODC Research, 2023). METH causes an increase in the stimulation of monoamine receptors by promoting the release and inhibiting the reuptake of monoamines such as dopamine and serotonin at neural synapses, resulting in a central stimulant effect (Cruickshank and Dyer, 2009). It causes loss of sleepiness, mood elevation, and euphoria, forming dependence. Repeated METH administration results in memory impairment and neurotoxicity (Shin et al., 2017). Rodent studies show reduced fertility, head circumference, deformities, and developmental delays in pups from METH-exposed mothers (Dong et al., 2018; Pometlová et al., 2009; Yamamoto et al., 1992). In humans, METH intake during pregnancy or lactation causes decreased head circumference, short stature, and malformations in newborns, and decreased memory and increased impulsivity in infants (Abar et al., 2014; Kiblawi et al., 2014; Piper et al., 2011; Smid et al., 2019). Therefore, administration of METH during pregnancy and lactation affects fetal and neonatal development.
Recently, the developmental origins of health and disease theory have attracted attention as a concept that environmental factors during embryonic and developmental stages shape health and disease in adulthood and old age. For example, maternal undernutrition, overnutrition, and alcohol consumption affect the risk of lifestyle-related diseases in offspring (Fall and Kumaran, 2019; Harper et al., 2014; Školníková et al., 2020). We previously reported that maternal exposure to methylphenidate (MPH), which is a first-line medicine for attention-deficit/hyperactivity disorder (ADHD), alters multiple gene expression levels in the striatum and induces ADHD-like phenotypes, such as hyperactivity and impulsivity, in the next generation (Aoki et al., 2021). More recently, the paternal origin of health and disease (POHaD) theory was advocated as a concept wherein paternal factors influence the next generation and environmental factors, such as obesity and excessive sugar ingestion (Craig et al., 2017). In addition, cocaine and Δ9-tetrahydrocannabinol (THC) induce depression-like behaviors and attention disorders in subsequent generations, respectively (Killinger et al., 2012; Levin et al., 2019). However, there are few reports on the use of pharmaceuticals or other chemicals. We previously reported that paternal MPH exposure increases impulsivity and impaired long-term memory in offspring, together with altered expression of ADHD-related genes (Nakano et al., 2023).
MPH and METH are structurally and pharmacologically similar, and both increase dopamine levels; however, the effects of METH are stronger than those of MPH. Therefore, paternal METH exposure might affect the next generation more than MPH. Most METH abusers are predominantly male, especially in Japan where approximately 80% of METH abusers are male (Ministry of Justice, 2022). However, the effects of paternal METH intake on offspring are unclear. This study determined whether paternal METH exposure alters offspring behavior and gene expression in mice. Several studies in rodents report that paternal factors affect the next generation (F1) and subsequent generations (F2 and beyond). Furthermore, paternal high-fat diet ingestion in rats affects the skeletal muscle of F2, but not F1 (Alm et al., 2017). Therefore, this study examined the impact on the next generation (F1) and the subsequent generation (F2).
Male and female mice were purchased from Sankyo Lab Service Co. (Tokyo, Japan). Mice were housed in plastic cages in a temperature-controlled room (22 ± 1°C) and maintained at a 12-hr light–dark cycle, with free access to food and water. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Showa University. Every effort was made to minimize the number of animals used and their suffering.
Drug administration for siresSix-week-old male ICR mice (F0) were administered 5 mg/10 mL/kg of METH (Sumitomo Pharma Co., Ltd., Osaka, Japan) or 10 mL/kg of saline (Otsuka Pharmaceutical, Tokyo, Japan) subcutaneously for 21 consecutive days. The day after the last administration, a male mouse was mated with a drug-untreated female mouse during its estrous cycle to obtain offspring (F1). Female mice were kept alone from the time of vaginal plug confirmation until delivery. Some 9-week-old male F1 mice were mated with untreated female mice to obtain the next offspring (F2). F1 and F2 derived from METH-administered sires were designated as the METH-F1 and METH-F2 groups, respectively. F1 and F2 derived from saline-administered sires were designated as control-F1 and control-F2 groups, respectively. In this study, we employed a 5 mg/kg dose of METH, since other animal studies with this drug used doses ranging from 1-20 mg/kg (Dong et al., 2018; Khoradmehr et al., 2015; Sabour et al., 2017; Yamamoto et al., 2002). In addition, we have shown that 5 mg/kg METH induces increases in locomotor activity and dopamine levels in the brain, and produces dependence on conditioned place preference test (Hataoka et al., 2017; Kaizaki et al., 2014a).
Time schedule of the experimentGrowth evaluation was performed on all F1 mice [control-F1 group (n=84) and METH-F1 group (n=103)]. F1 mice (6 weeks old) were randomly divided into four groups (Fig. 1A). Group F1-A [control-F1 group (n=16) consisting of eight males and eight females, and METH-F1 group (n=16) consisting of 8 males and 8 females] was subjected to a spontaneous locomotor activity test at 6 weeks of age and an acute METH treatment test the following day. Group F1-B [control-F1 group (n=30) consisting of 15 males and 15 females and METH-F1 group (n=30) consisting of 15 males and 15 females] was subjected to a spontaneous locomotor activity test, an elevated plus maze (EPM) test at 6 weeks of age, and a passive avoidance test at 7 weeks of age. Brain samples from group F1-C [control-F1 group (n=6) consisting of males and METH-F1 group (n=5) consisting of males] were collected at 7 weeks of age. Groups F1-D [control-F1 group (n=7) and METH-F1 group (n=7)] only consisted of male mice and were mated with untreated female mice to obtain F2 at 9 weeks of age.
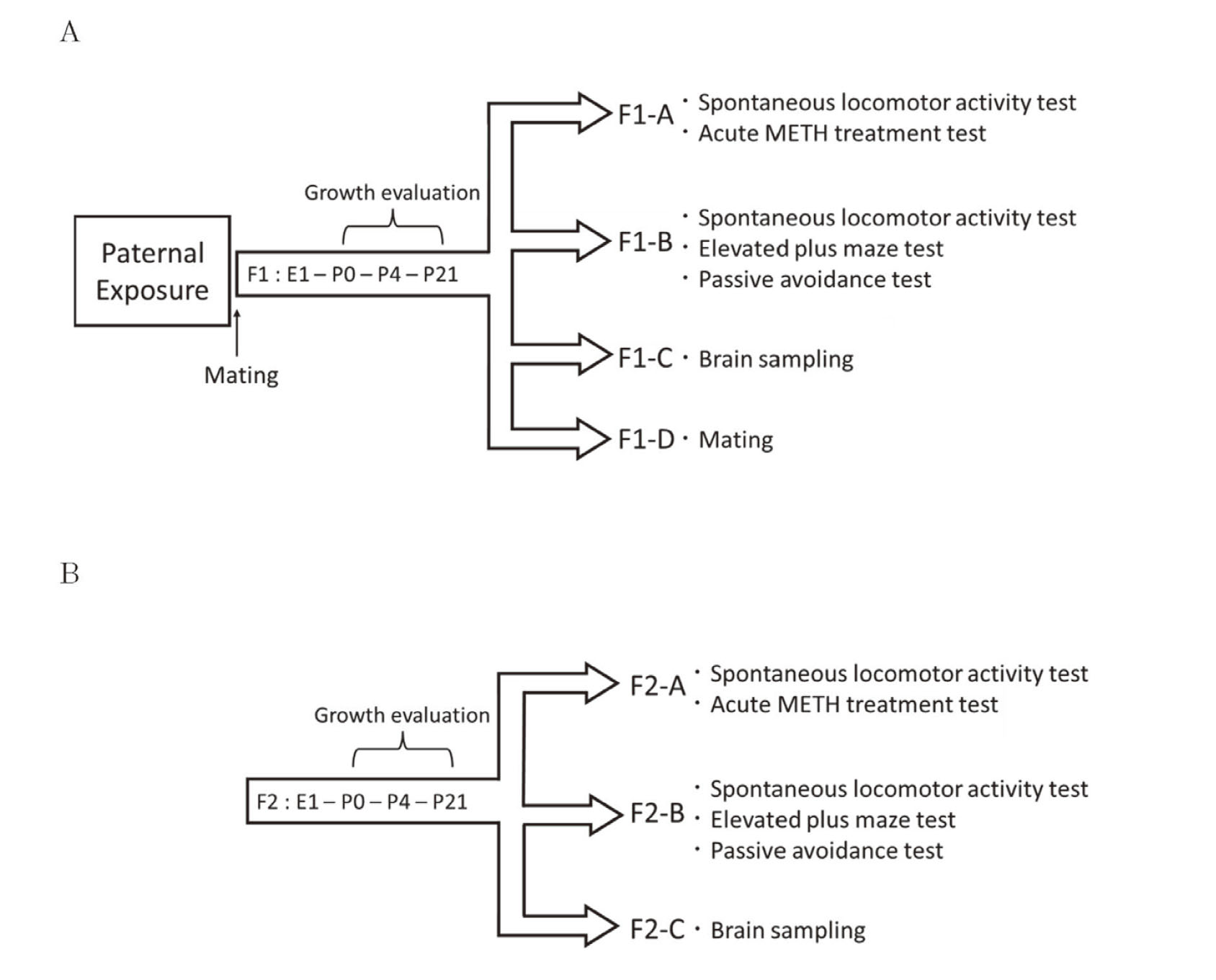
Experimental design of this study. (A) Sires were subcutaneously administered METH (5 mg/kg) or saline (10 mL/kg) for 21 consecutive days. The sires were mated with drug-untreated female mice the day after the last administration, and offspring (F1) were obtained. Growth evaluation was performed on all F1 mice. F1 mice were divided into 4 groups at 6 weeks of age. Group F1-A was subjected to a spontaneous locomotor activity test and an acute METH treatment test. Group F1-B was subjected to a spontaneous locomotor activity test, an elevated plus maze test, and a passive avoidance test. Group F1-C had brain samples taken at 7 weeks of age. Group F1-D only consisted of male mice and were mated with untreated females at 9 weeks of age. (B) Growth evaluation was performed on all F2 mice. F2 mice were divided into 3 groups at 6 weeks of age. Groups F2-A, B, and C were subjected to the same tests as that of groups F1-A, F1-B, and F1-C, respectively.
Growth was evaluated in all F2 mice [control-F2 group (n=91) and METH-F2 group (n=94)], which were randomly divided into three groups at 6 weeks of age (Fig. 1B). Groups F2-A [control-F2 group (n=16) consisting of 8 males and 8 females, and METH-F2 group (n=16) consisting of 8 males and 8 females], F2-B [control-F2 group (n=30) consisting of 15 males and 15 females, and METH-F2 group (n=30) consisting of 15 males and 15 females], and F2-C [control-F2 group (n=7) consisting of males and METH-F2 group (n=7) consisting of males] were subjected to the same tests as F1-A, F1-B, and F1-C, respectively.
Growth evaluationThe day of birth was designated as postnatal day (P) 0. The number of offspring was counted at P1 and the survival rate was evaluated at P4. Body weights were measured daily from P4 to P21. The tests for growth evaluation were based on modifications reported by Kaizaki et al. (2014b).
Righting reflex testThis test is believed to be a reflection of muscle strength and subcortical maturation. Offspring were placed on their backs, and the time to turn over on all four paws and touch the platform was measured. The maximum time permitted was 60 sec. The number of offspring with successful responses in less than 2 sec was recorded. This test was carried out every 2 days from P4 to P12.
Cliff avoidance testThis test was used to assess the integration of exteroceptive input (vibrissae) and locomotor output. Offspring placed on the edge of a platform (W23 cm × D30 cm × H20 cm) with forepaws and chests extending over the edge tend to move away by backing up or turning to the side. Avoidance was scored by reflex latency between being placed on the edge and turning the body sideways with forepaws or turning the head away. If the offspring did not make respond within 60 sec or fell off the platform, the latency was recorded as 60 sec. This test was carried out every 3 days from P9 to P18.
Wire-hanging maneuverThis test was used to assess neuromuscular and locomotor development. Offspring suspended by their forepaws from a horizontal wire (1.6 mm in diameter, 55 cm long, 30 cm high between two poles) tend to support themselves with their hind paws to avoid falling and to aid in progression along the rod. The latency to fall down and the ability to grip the wire was scored as follows: score 0, offspring falls immediately; score 1, grips the wire with forelimbs; score 2, grips the wire with forepaws and tries to support itself with its hind paws; score 3, grips the wire with 3 or 4 paws; score 4, grips the wire with 4 paws and twists its tail around the wire; score 5, grips the wire with 4 paws, twist its tail around the wire, and moves to the pole. The maximum time permitted was 60 sec. If the offspring moved to the pole within 60 sec, the latency time was recorded as 60 sec. This test was carried out every 3 days from P10 to P19.
Behavioral test in adolescence Spontaneous locomotor activity testA spontaneous locomotor activity test was performed to assess hyperactivity. Six-week-old mice were placed in activity chambers (W20 cm × D20 cm × H25 cm) for 20 min to acclimatize to the environment. Then the locomotor activity was monitored using a video camera mounted on the ceiling for analysis. The mice were placed in activity chambers, and the total distance traveled was measured for 70 min. Locomotor activity was measured using the ANY-maze video tracking software (Stoelting Co., Wood Dale, IL, USA).
Acute METH treatmentAn acute treatment test was performed the day after the spontaneous locomotor activity test to examine the sensitivity to METH. Mice were placed in an activity chamber and acclimated for 35 min, followed by measuring basal locomotor activity for 15 min. Subsequently, 5 mg/kg METH was subcutaneously administered and locomotor activity was measured for 35 min.
Elevated plus maze testAn EPM test, which is used to assess anxiety behavior (Schmitt et al., 2002), was performed as previously described (Wang et al., 2013) with modifications. The plus maze consists of two open arms (30 cm × 6 cm × 0.5 cm) and two closed arms (30 cm × 6 cm × 20 cm) emanating from a common central platform (6 cm × 6 cm) to form a plus shape. The entire apparatus was elevated to a height of 40 cm above the floor. A video camera and illumination lamps were mounted on the ceiling. Anxiety-related behaviors for each mouse were recorded for 10 min. At the beginning of the test, the mouse was placed on the central platform with its head facing an open arm. The parameters recorded included the numbers of open arm or closed arm entries (arm entry was defined as 80% of the body entering the arm; if the body did not enter open arm or closed arm, it was defined as entering the central platform), the total time that each mouse spent in various sections of the maze (open arms and closed arms), and the distance that each mouse traveled in open arms and closed arms. The ANY-maze video tracking software was used to analyze the data. The data from mice that fell from the platform during measurements were excluded from the analysis.
Passive avoidance testPassive avoidance involves the learned inhibition of natural response and provides information on learning and memory abilities (Baarendse et al., 2008). The passive avoidance procedure consists of two sessions including the learning and the memory trials (Nakano et al., 2023). The apparatus used in the step-through type of passive avoidance protocol consisted of two compartments [one lit compartment (W19 cm × D24 cm × H20 cm) and one dark compartment (W19.5 cm × D14 cm × H19 cm)], connected via a door. In the learning trial, each mouse was placed in the lit compartment and allowed to freely enter the dark compartment through the door. Once the mouse entered the dark compartment, an electrical foot shock was immediately delivered (1 sec, 0.6 mA) using Passive Avoidance Control (O'Hara Co., Tokyo, Japan). Although mice repeatedly stepped through the door, they eventually remained in the lit compartment. The number of electrical foot shocks required to hold the mouse in the lit compartment for 5 min was recorded as a measure of the acquisition of passive avoidance. The memory trial was carried out 24 hr after the learning trial. The mouse was placed in the lit compartment. The dark compartment was not connected to the electric shock generator. As a measure of passive avoidance retention, we recorded whether each mouse entered the dark compartment during 5 min.
RNA expression levelsThe striatum of brains was collected from 7 weeks old male offspring (control-F1, n=6; METH-F1 n=5; control-F2, n=7; METH-F2, n=7), quickly frozen in liquid nitrogen, and stored at -80°C until total RNA was extracted. Total RNA was isolated from the tissue using RNeasy Mini Kit (Qiagen Inc., CA, USA).
RNA-seq analysisTotal RNA samples collected from the striatum of 7 weeks old offspring (control-F1, n=4; METH-F1 n=3; control-F2, n=4; METH-F2, n=4) were submitted to Macrogen Japan (Tokyo, Japan) for bioanalyzer quality control analysis, Illumina next-generation sequencing, and differential expressed gene (DEG) analysis. All submitted samples had an RNA integrity number > 7 (on a scale of 1–10, with 10 meaning the best quality sample with the least advanced degradation) and proceeded for library construction. Sequencing libraries were prepared from poly-A selected RNA of each sample using the TruSeq Stranded mRNA Library Prep Kit (Illumina Inc., CA, USA). Transcriptome sequencing (100 bp paired-end sequencing) was performed on a NovaSeq 6000 System (Illumina Inc.). Adapter sequences and low-quality bases in paired-end reads were removed with Trimmomatic (version 0.38). Filtered paired-end reads were mapped to the mouse reference genome (mm10) by HISAT2 (version 2.1.0) and expression levels were quantified by StringTie (version 2.1.3b). Statistical analysis was performed by DESeq2 to identify DEGs. DEGs were extracted by using the following criteria: fold-change (FC) ≥ 1.2, p-value < 0.05, and average read counts ≥ 100 for either the control or METH group. Raw and processed data are available in the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo/) under accession number GSE248542. Identified DEGs were illustrated as a clustered heat map using Heatmapper (http://www.heatmapper.ca/).
Bioinformatics analysisMetascape (https://metascape.org/gp/index.html) was used for enrichment analysis (Zhou et al., 2019). The DEG list containing 337 DEGs in the METH-F1 group and 355 DEGs in the METH-F2 group was entered into the Metascape platform and the key functional terms were analyzed. The process of converting the DEG list to the corresponding Entrez gene ID of M. musculus and the list of annotations was the latest version of the database at the time of analysis. Terms with a p-value < 0.01, a minimum count of 3, and an enrichment factor > 1.5 (the enrichment factor is the ratio between the observed counts and the counts expected by chance) are collected and grouped into clusters based on their membership similarities.
Quantitative reverse transcription-PCR (qRT-PCR)cDNA was synthesized from the extracted RNA using PrimeScript RT Master Mix (Takara Bio Inc., Shiga, Japan). Real-time PCR with a FAM-labeled probe for the target cDNA and a VIC-labeled probe for the housekeeping gene was performed using TaqMan Fast Advanced Master Mix (Applied Biosystems, CA, USA) on a StepOne real-time PCR system (Applied Biosystems) or QuantStudio 3 (Applied Biosystems) according to the manufacturer’s protocol. PCR was performed in two steps. Amplification conditions were 95°C for 20 sec in the hold stage, followed by 40 cycles of 95°C for 1 sec and 60°C for 20 sec in the PCR stage. mRNA levels were measured as the relative ratio to β-actin mRNA. All predesigned PCR primers and TaqMan MGB probes were purchased from Applied Biosystems. The assay IDs of the probes used in this study were as follows: mouse Cartpt, Mm04210469_m1; mouse Gabrg2, Mm00433489_m1; mouse Gabra5, Mm00621092_m1; mouse Stx1a, Mm00444008_m1; mouse Syt1, Mm00436858_m1.
Statistical analysisSurvival rate at P1, body weight at P4, righting reflex, scores, and times in the wire-hanging maneuver, cliff avoidance test, and passive avoidance test were analyzed using the Wilcoxon rank-sum test. The rate of weight gain was analyzed using repeated-measures analysis of variance (ANOVA). The success rates of the righting reflex and cliff avoidance were analyzed using the chi-square test. The number of births, spontaneous locomotor activity, EPM, and mRNA expression data were analyzed using Student's t-test. Data of RNA-seq was analyzed using DESeq2 to identify DEGs. The acute METH treatment test data were analyzed using Welch's t-test. Statistical analyses were performed with JMP Pro 15.0.0 (SAS Institute, Cary, NC, USA).
We evaluated the number of births at P1, neonatal survival rate at P4, and body weight at P4 to determine the effect of paternal exposure to METH on fetal growth. There were no significant differences in the number of births, survival rates, or body weights between the control-F1 group and the METH-F1 group (Table 1). Additionally, no external abnormalities such as deformities were observed (data not shown). During infancy, body weight was measured to assess physical development, the righting reflex test was used to assess muscle strength and subcortical maturation, the cliff avoidance test was used to assess the integration of exteroceptive input (vibrissae) and locomotor output, and the wire-hanging maneuver was used to assess neuromuscular and locomotor development. The rate of weight gain in the METH-F1 group until weaning (P21) was significantly lower than that of the control-F1 group (Fig. 2). The latency time from P4 to P8 in the righting reflex test of the METH-F1 group was significantly longer than that in the control-F1 group (Fig. 3A). The success rate of the righting reflex at P8 was 56.0% in the control-F1 group and 32.0% in the METH-F1 group, which was significantly lower than that in the METH-F1 group (data not shown). However, no significant differences were observed in the latency time or success rate between the two groups after P10. The latency time in the cliff avoidance test of the METH-F1 group was significantly longer than that of the control-F1 group from P9 to P12 (Fig. 3B). The cliff avoidance rates at P12 were 85.7% in the control-F1 group and 67.0% in the METH-F1 group, and were significantly lower in the METH-F1 group (data not shown). After P15, there was no difference in the latency time or avoidance rate between the two groups. The scores of the METH-F1 group in the wire-hanging maneuver were significantly lower than those of the control-F1 group during the entire study period (P10-P19, Fig. 3C). However, there was no significant difference in latency time between the two groups (Fig. 3D). There were no differences between the sexes in the results of these growth evaluations (data not shown).
| Control | METH | |||||
|---|---|---|---|---|---|---|
| Number of births | 14.5 | ± | 1.06 | 14.7 | ± | 0.68 |
| Survival rate on P4 (%) | 97.8 | ± | 1.36 | 100 | ± | 0 |
| Weight on P4 (g) | 3.2 | ± | 0.05 | 3.2 | ± | 0.04 |
Offspring were counted on postnatal day 1 (P1). The number of offspring includes live and stillborn offspring. The survival rate on P4 was calculated by dividing the number of survivors on P4 by the number of offspring on P1. Data are shown as the mean ± S.E. [Control dams (n=6, females mated with saline-administrated males), METH dams (n=7, females mated with METH-administrated males).]
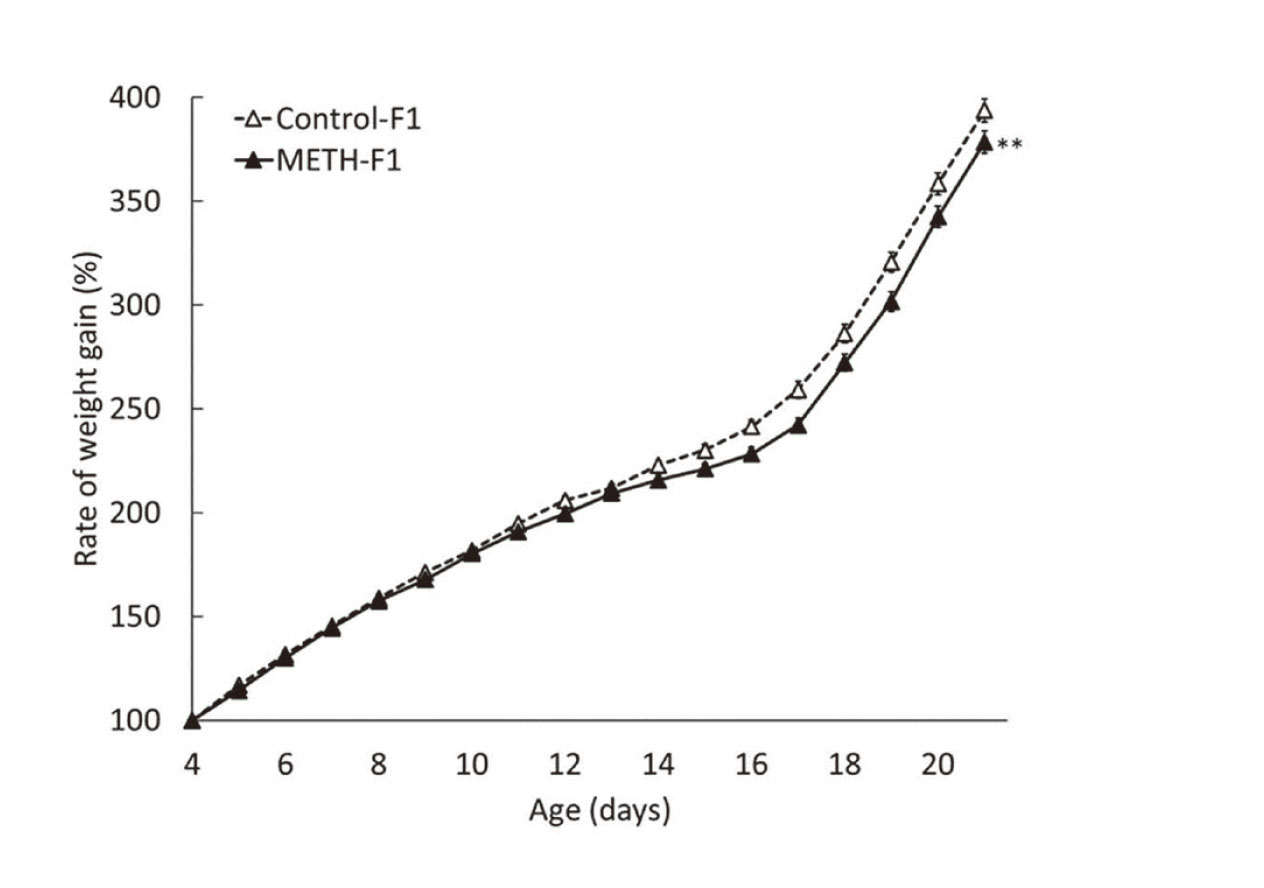
Effects of METH administration to sires on body weight in F1 mice. Body weight was measured from P4 to P21. The weight of P4 was set to 100%, and the rate of weight gain to P21 is shown in %. Data are shown as mean ± S.E. and analyzed using repeated-measures ANOVA. Control-F1, n=84; METH-F1, n=103. **p < 0.01 vs the control-F1.

Effects of METH administration to sires on growth in F1 mice. (A) Righting reflex latencies of offspring aged P4 to P12 were measured with a 60-sec cut-off time. (B) The latencies of offspring aged P9 to P18 were measured with a 60-sec cut-off time. (C) The ability to grip the wire in the wire-hanging maneuver was scored. (D) The latency time was measured with a 60-sec cut-off time. Data are shown as mean ± S.E. and analyzed using the Wilcoxon rank-sum test. Control-F1, n=84; METH-F1, n=103. *p < 0.05, **p < 0.01 vs the control-F1.
Locomotor activity was measured to assess the effects of paternal METH exposure on the spontaneous locomotor activity and sensitivity to METH in F1 mice. There was almost no significant difference in locomotor activity between the two groups in male mice (Fig. 4A); however, locomotor activity tended to decrease in the METH-F1 group in female mice, especially in the latter half of the measurement period (Fig. 4B). Locomotor activity in the acute METH treatment tests immediately increased after METH administration in all groups, peaking approximately 20 min after administration (Fig. 5). There was no significant difference in the locomotor activity after METH administration between the METH-F1 and control-F1 groups in either males or females. EPM tests were used to examine the effects of paternal METH exposure on anxiety-related behaviors. The number of entries, time spent, and distance traveled in each arm were similar between the METH-F1 and control-F1 groups in both males and females (Fig. 6). Passive avoidance tests were performed to examine the effects of paternal METH exposure on the memory of F1 mice. The number of electrical foot shocks in the learning trial was similar between the METH-F1 and control-F1 groups in males, but significantly greater in the METH-F1 group than in the control-F1 group in females (Fig. 7A). In contrast, the retention latency in the memory trial was significantly shorter in the METH-F1 group than in the control-F1 group in males, but there was no significant difference between the two groups in females (Fig. 7B).
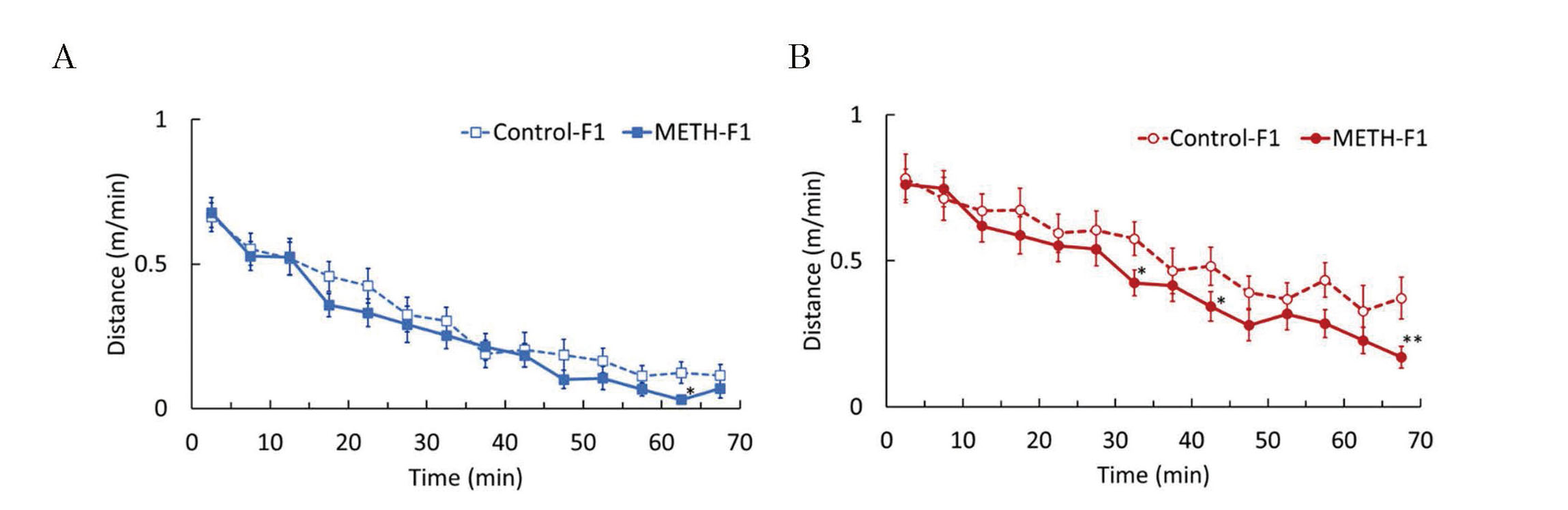
Effects of METH administration to sires on spontaneous activity in F1 mice. A spontaneous locomotor activity test was performed at 6 weeks of age. Graphs were plotted with the average distance traveled over a 5-min period during the measurement. The graphs represent F1 males (A) and F1 females (B). Data are shown as mean ± S.E. and analyzed using Student's t-test. Control-F1 (male), n=23; METH-F1 (male), n=23; control-F1 (female), n=22; METH-F1 (female), n=23. *p < 0.05, **p < 0.01 vs each control-F1.

Effects of METH administration to sires on susceptibility to METH in F1 mice. The acute METH treatment test was performed at 6 weeks of age. Basal locomotor activity was measured for 15 min and the mice were administered METH (5 mg/kg). Locomotor activity was measured 35 min after administration. Graphs were plotted with the average distance traveled over a 5-min period during the measurement. The graphs represent F1 males (A) and F1 females (B). Data are shown as mean ± S.E. and analyzed using Student's t-test. Control-F1 (male), n=8; METH-F1 (male), n=8; control-F1 (female), n=8; METH-F1 (female), n=8.
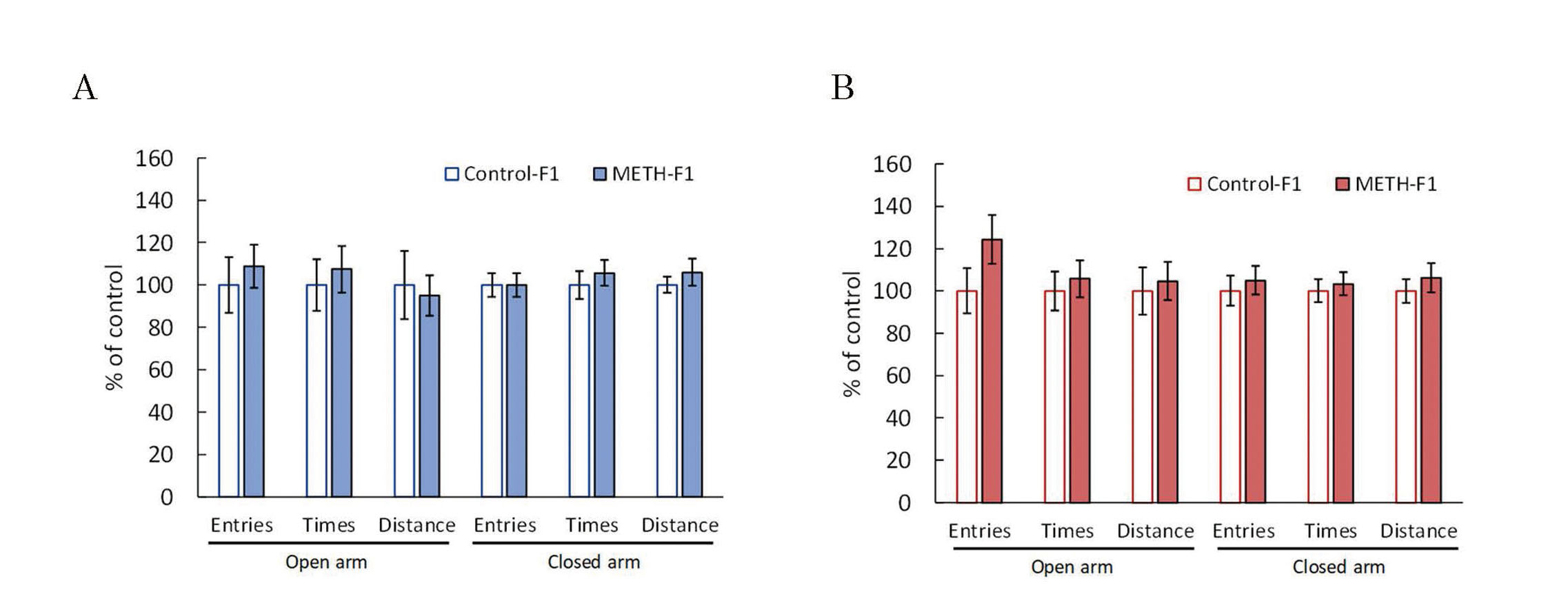
Effects of METH administration to sires on anxiety behavior in F1 mice. The elevated plus maze test was performed at 6 weeks of age. This test was analyzed using a video tracking system to determine the number of entries, time spent, and distance traveled by each open arm and closed arm. The graphs represent F1 males (A) and F1 females (B). Data are shown as mean ± S.E. and analyzed using the Wilcoxon rank-sum test. Control-F1 (male), n=14; METH-F1 (male), n=15; control-F1 (female), n=14; METH-F1 (female), n=13.
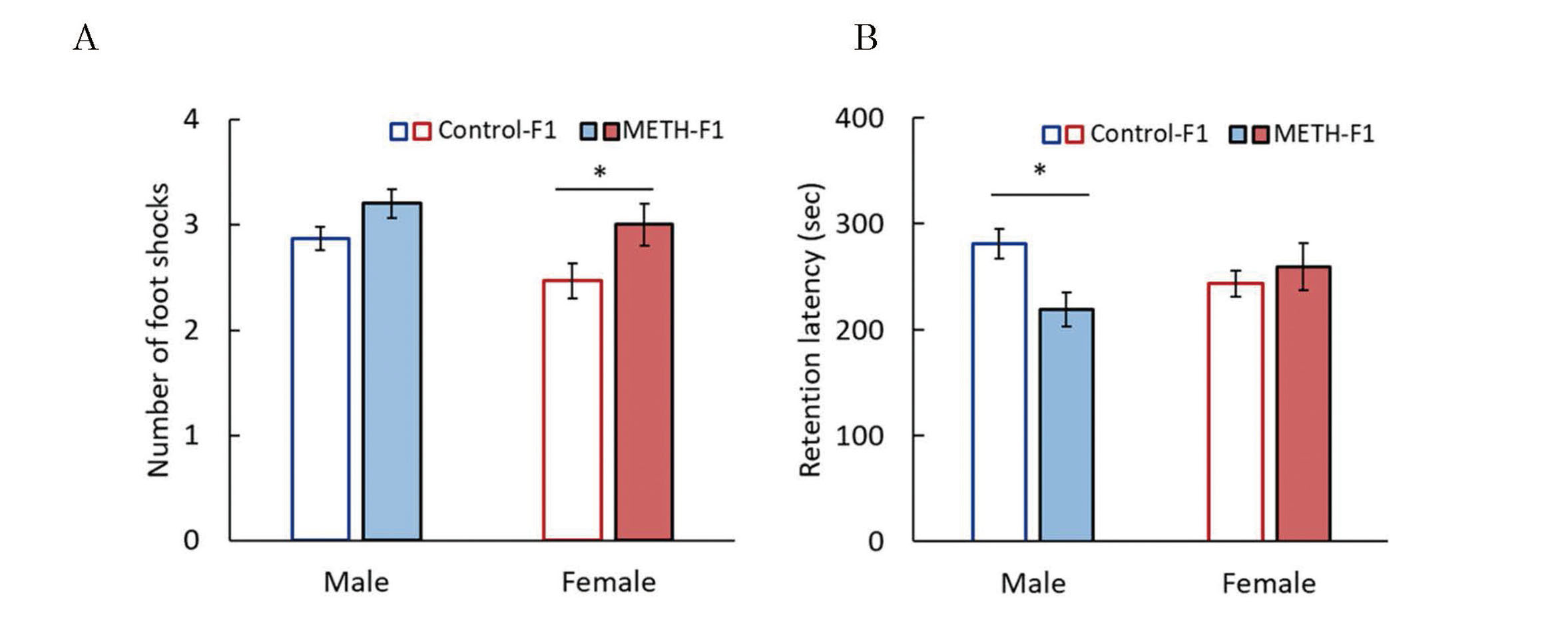
Effects of METH administration to sires on learning and memory abilities in F1 mice. The passive avoidance test was performed at 7 weeks of age. The number of electrical foot shocks required to retain the mouse in the lit compartment for 5 min was recorded as a measure of acquisition of passive avoidance (A), and the retention latency time was recorded 24 hr after the learning trial as an index of passive avoidance retention (B). Data are shown as mean ± S.E. and analyzed using the Wilcoxon rank-sum test. Control-F1 (male), n=15; METH-F1 (male), n=15; control-F1 (female), n=15; METH-F1 (female), n=15. * p < 0.05 vs the control-F1.
The main target site of METH is the striatum. Therefore, RNA-seq analysis was performed in male F1 mice to examine the effects of METH on gene expression in the striatum. The striatum showed 337 DEGs in the METH-F1 group (181 and 156 showing increased and decreased expression, respectively) (Figs. 8A and B). Enrichment analysis with Metascape showed significant enrichment of pathways and gene ontologies (GOs) related to behavior and neurology, such as "behavior" and "neuron projection development" (Fig. 8C). Among the "behavior" consisting of 43 genes, we focused on Cartpt (FC -2.3), Gabrg2 (FC -1.2), and Gabra5 (FC -1.4). Since Cartpt is considered to be directly related to methamphetamine, and Gabrg2 and Gabra5 are GABA receptor-related genes. Gabrg2 was also variable in our previous study (Nakano et al., 2023). We also focused on genes related to exocytosis (Syt1, Stx1a) that were altered in our previous study (Nakano et al., 2023). Gene expression levels in Cartpt, Gabrg2, and Gabra5 of the METH-F1 group were 73.7% (p-value=0.22), 83.2% (p-value =0.09), and 85.6% (p-value=0.15), respectively (Fig. 9). Syt1 and Stx1a expression levels were comparable in the METH-F1 and the control-F1 groups (Fig. 9).

Identification of DEGs and enrichment analysis in the striatum of F1 male mice. Total RNA collected from the offspring striatum at 7 weeks of age was subjected to RNA-seq to identify DEGs (control-F1, n=4; METH-F1, n=3). DEGs were subjected to enrichment analysis using Metascape. (A) Volcano plot of 13449 genes with average read counts ≥ 100 for either the control-F1 or METH-F1 group. Genes that pass a threshold of fold change ≥ 1.2, p-value < 0.05 are highlighted by yellow (up-regulated genes) and blue (down-regulated genes), containing 181 and 156 DEGs, respectively. (B) Clustering heat map of 337 DEGs in F1 mice. The vertical and horizontal axes represent the samples and the DEGs, respectively. (C) The top 20 enriched ontology clusters were created by enrichment analysis using Metascape and color-coded by p-value. The horizontal axis represents the −log10(p). Names of gene ontology and Reactome terms are listed on the right panel.

Gene expression of Cartpt, Gabrg2, Gabra5, Stx1a and Syt1 in the striatum of F1 male mice. Brain striatum samples from F1 mice were obtained at 7 weeks of age. Gene expression was determined by quantitative RT-PCR. Data are shown as mean ± S.E. and analyzed using Student's t-test. Control-F1, n=6; METH-F1, n=5.
There was no difference in the number of births at P1 and the neonatal survival rate at P4 between the control-F2 and METH-F2 groups (Table 2), and no external abnormalities such as deformities were observed (data not shown). In contrast, the body weight at P4 in the METH-F2 group was significantly higher than that in the control-F2 group (Table 2), and the rate of weight gain in the METH-F2 group until weaning (P21) was significantly higher than that in the control-F2 group (Fig. 10). The latency time from P4 to P6 in the righting reflex test of the METH-F2 group was significantly longer than that in the control-F2 group, whereas no significant difference was observed in latency time after P8 (Fig. 11A). The success rate of the righting reflex at P6 was significantly lower in the METH-F2 group (11.7%) compared with 35.2% in the control-F2 group (data not shown). There was no significant difference in latency time between the two groups during the entire study period (P9-P18) in the cliff avoidance test (Fig. 11B). The cliff avoidance rates at P12 were not significantly different between the METH-F2 group (88.3%) and the control-F2 group (94.5%) (data not shown). There was no significant difference in latency time between P10 and P13 in the wire-hanging maneuver, but the scores in the METH-F2 group were significantly lower than those in the control-F2 group (Figs. 11C and D). There were no differences between the sexes in the results of these growth evaluations (data not shown).
| Control | METH | |||||
|---|---|---|---|---|---|---|
| Number of births | 13.0 | ± | 0.69 | 13.6 | ± | 0.57 |
| Survival rate on P4 (%) | 100 | ± | 0 | 99.0 | ± | 0.95 |
| Weight on P4 (g) | 3.2 | ± | 0.04 | 3.3 | ± | 0.05* |
Offspring were counted on postnatal day 1 (P1). The number of offspring includes live and stillborn offspring. The survival rate on P4 was calculated by dividing the number of survivors on P4 by the number of offspring on P1. Data are shown as the mean ± S.E. *p < 0.05 vs the control-F2. [Control dams (n=7, females mated with control-F1 males), METH dams (n=7, females mated with METH-F1 males).]

Effects of METH administration to sires on body weight in F2 mice. Body weight was measured from P4 to P21. The weight of P4 was set to 100%, and the rate of weight gain to P21 is shown in %. Data are shown as mean ± S.E. and analyzed using repeated-measures ANOVA. Control-F2, n=91; METH-F2, n=94. **p < 0.01 vs the control-F2.

Effects of METH administration to sires on growth in F2 mice. (A) Righting reflex latencies of offspring aged P4 to P12 were measured with a 60-sec cut-off time. (B) The latencies of offspring aged P9 to P18 were measured with a 60-sec cut-off time. (C) The ability to grip the wire in the wire-hanging maneuver was scored. (D) The latency time was measured with a 60-sec cut-off time. Data are shown as mean ± S.E. and analyzed using the Wilcoxon rank-sum test. Control-F2, n=91; METH-F2, n=94. *p < 0.05, **p < 0.01 vs the control-F2.
The activity of the male METH-F2 group was similar to that of the control-F2 group in spontaneous locomotor activity tests (Fig. 12A). In contrast, the activity of the METH-F2 group was significantly lower than that of the control-F2 group approximately 30 min after the start of the test in females (Fig. 12B). Locomotor activity in the acute METH treatment tests immediately increased after METH administration in all groups, peaking approximately 20 min after administration (Fig. 13). Locomotion in the METH-F2 group was significantly lower than that in the control-F2 group immediately after administration to 10 min after administration in males and from 5 to 15 min after administration in females (Fig. 13). The EPM tests showed that there were no significant differences between the METH-F2 and control-F2 groups for any of the evaluation items in males (Fig. 14A). In contrast, the time spent and distance traveled in the open arm of the METH-F2 group significantly increased in females, and the number of entries into the closed arm significantly decreased compared to that in the control-F2 group (Fig. 14B). The number of electrical foot shocks in the learning trial and the retention latency in the memory trial in the passive avoidance tests were not significantly different between the METH-F1 and control-F1 groups for either sex (Fig. 15).

Effects of METH administration to sires on spontaneous locomotor activity in F1 mice. A spontaneous locomotor activity test was performed at 6 weeks of age. Graphs were plotted with the average distance traveled over a 5-min period during the measurement. The graphs represent F2 males (A) and F2 females (B). Data are shown as mean ± S.E. and analyzed using Student’s t-test. Control-F2 (male), n=23; METH-F2 (male), n=23; control-F2 (female), n=23; METH-F2 (female), n=23. *p < 0.05 vs each control-F2.
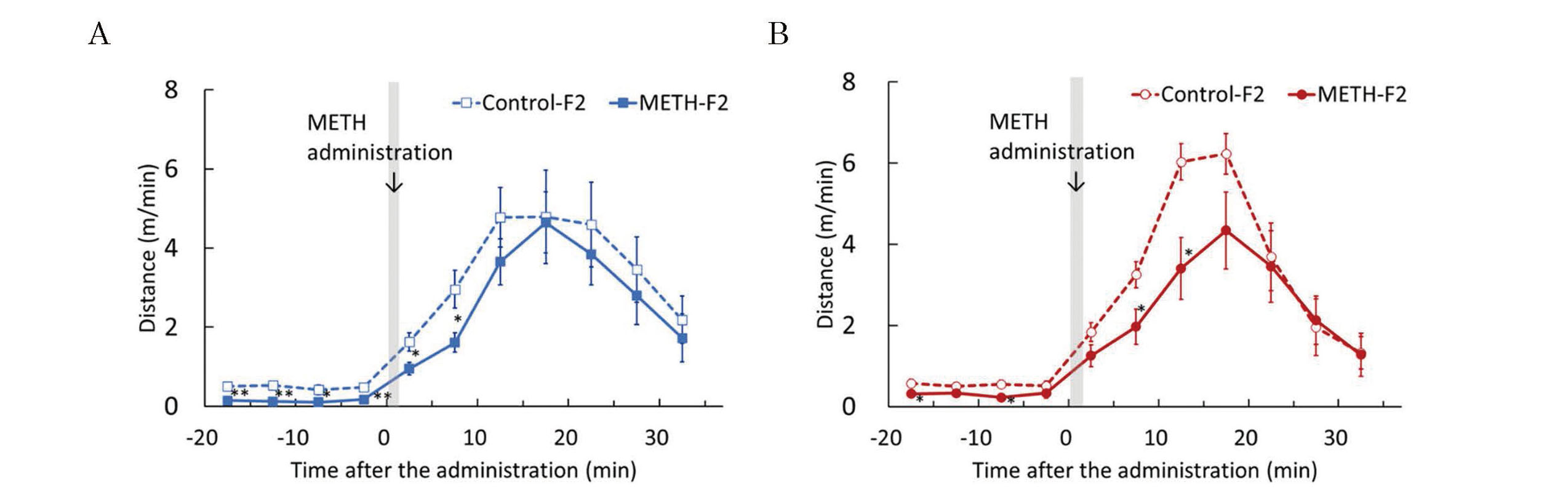
Effects of METH administration to sires on susceptibility to METH in F2 mice. The acute METH treatment test was performed at 6 weeks of age. Basal locomotor activity was measured for 15 min and the mice were administered METH (5 mg/kg). Locomotor activity was measured 35 min after administration. Graphs were plotted with the average distance traveled over a 5-min period during the measurement. The graphs represent F2 males (A) and F2 females (B). Data are shown as mean ± S.E. and analyzed using Student’s t-test. Control-F2 (male), n=8; METH-F2 (male), n=8; control-F2 (female), n=8; METH-F2 (female), n=8. *p < 0.05, **p < 0.01 vs each control-F2.

Effects of METH administration to sires on anxiety behavior in F2 mice. The elevated plus maze test was performed at 6 weeks of age. This test was analyzed using a video tracking system to determine the number of entries, time spent, and distance traveled by each open arm and closed arm. The graphs represent F2 males (A) and F2 females (B). Data are shown as mean ± S.E. and analyzed using the Wilcoxon rank-sum test. Control-F2 (male), n =15; METH-F2 (male), n=15; control-F2 (female), n =15; METH-F2 (female), n=15. *p < 0.05, **p < 0.01 vs each control-F2.
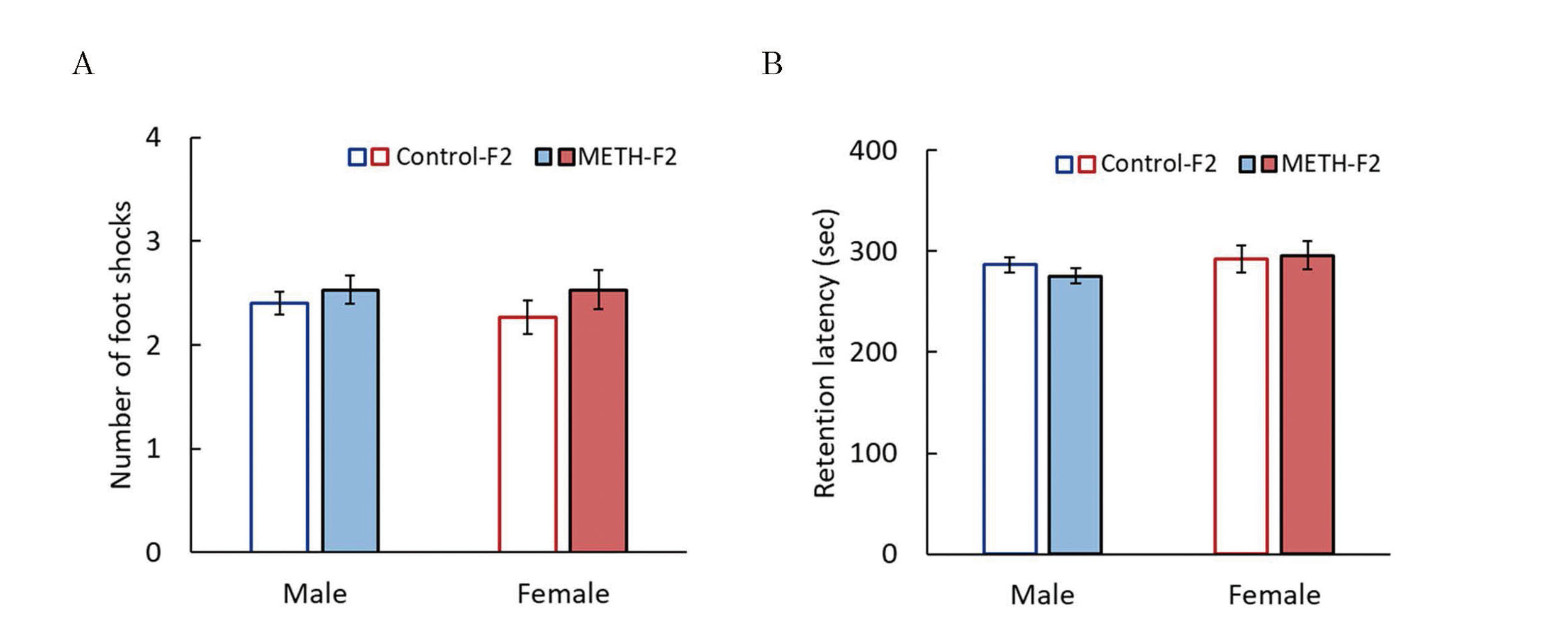
Effects of METH administration to sires on learning and memory abilities in F2 mice. The passive avoidance test was performed at 7 weeks of age. The number of electrical foot shocks required to retain the mouse in the lit compartment for 5 min was recorded as a measure of acquisition of passive avoidance (A), and the retention latency time was recorded 24 hr after the learning trial as an index of passive avoidance retention (B). Data are shown as mean ± S.E. and analyzed using the Wilcoxon rank-sum test. Control-F2 (male), n=15; METH-F2 (male), n=15; control-F2 (female); n=15, METH-F2 (female), n=15.
Analysis of gene expression changes in the striatum revealed that 355 genes were altered in the METH-F2 group (140 and 215 showed increased and decreased expression, respectively) (Figs. 16A and B). Enrichment analysis with Metascape showed significant enrichment of GOs related to essential parts of the organism such as "protein folding" and "protein processing in the endoplasmic reticulum" (Fig. 16C).

Identification of DEGs and enrichment analysis in the striatum of F2 male mice. Total RNA collected from the offspring striatum at 7 weeks of age was subjected to RNA-seq to identify DEGs (control-F2, n=4, and METH-F2, n=4). DEGs were subjected to enrichment analysis using Metascape. (A) Volcano plot of 13393 genes with average read counts ≥ 100 for either the control-F2 or METH-F2 group. Genes that pass a threshold of fold change ≥ 1.2, p-value < 0.05 are highlighted by yellow (up-regulated genes) and blue (down-regulated genes), containing 140 and 215 DEGs, respectively. (B) Clustering heat map of 355 DEGs in F2 mice. The vertical and horizontal axes represent the samples and the DEGs, respectively. (C) The top 20 enriched ontology clusters were created by enrichment analysis using Metascape and color-coded by p-value. The horizontal axis represents the −log10(p). Names of gene ontology and Reactome terms are listed on the right panel.
This study aimed to clarify the effects of paternal METH exposure on growth during infancy, behavior, and gene expression in F1 and F2 mice. In particular, we focused on whether there were similarities between the effects of METH and those of MPH, as we have previously reported (Nakano et al., 2023).
The number of births at P1, neonatal survival rate at P4, and body weight at P4 in the METH-F1 group were comparable to those in the control-F1 group, with no significant differences (Table 1). Maternal exposure to METH results in a shorter gestation period and lower birth weight (Yamamoto et al., 1992), although it does not affect the number of births or survival rates (Dong et al., 2018; Khoradmehr et al., 2015). This study observed no reduction in the gestational period or birth weight, suggesting that this may have resulted from fetal METH exposure. Although METH administration at the comparative doses with this study has been reported to reduce sperm motility (Sabour et al., 2017) and produce apoptosis in spermatogonium cells in the seminiferous tubules (Yamamoto et al., 2002), we found that paternal METH exposure did not affect the number of births and survival rates under current experimental conditions. This suggests that a slight decrease in sperm volume or sperm motility does not significantly affect fertilization ability. The rate of weight gain from P4 to P21 was significantly lower in the METH-F1 group than that in the control-F1 group (Fig. 2), suggesting that paternal METH exposure may affect feeding behavior or energy metabolism in F1 mice during infancy. Our previous study only observed the effect of paternal MPH exposure on the body weight of F1 mice approximately two weeks after birth (Nakano et al., 2023). This suggests that paternal METH exposure may affect the rate of weight gain for a longer period than MPH exposure. The results of the righting reflex test, cliff avoidance test, and wire-hanging maneuver (Fig. 3) showed developmental delays in muscle strength and the integration of external receptive inputs and motor outputs in METH-F1 mice. However, they were generally comparable to the control-F1 mice at the time of weaning. This indicated that the effect of paternal exposure to METH on developmental delay is limited.
Spontaneous locomotor activity was measured to assess adolescent behavior. A decrease in spontaneous locomotor activity was observed in the METH-F1 group at certain time points (Fig. 4). However, there was no significant difference in the total distance traveled during the measurement period. This indicated that paternal METH exposure did not affect spontaneous locomotor activity in F1 mice. Furthermore, there was no significant difference in locomotor activity after METH administration in the acute METH treatment test between the METH-F1 and control-F1 groups in male or female mice (Fig. 5). This indicated that paternal METH exposure did not affect METH sensitivity in F1 mice.
The EPM test was developed to evaluate the effects of anxiolytic drugs in rats and was later used to assess impulsivity in mice (Lister, 1987; Pellow et al., 1985; Ueno et al., 2002). The greater the number of entries into the open arm, the time spent in the open arm, and the distance traveled in the open arm in a mouse, indicating that they are less anxious and more impulsive. Methamphetamine administration decreases anxiety in mice (Miladi-Gorji et al., 2015), but there were no significant differences between the METH-F1 and control-F1 groups for each evaluation item of the EPM test (Fig. 6). Miladi-Gorji et al. (2015) and our findings indicate that the anxiety-reducing effect of METH occurs in administered mice and paternal METH exposure does not affect anxiety-like behavior in F1 mice.
Learning trials in the passive avoidance test can evaluate learning ability, and memory trials can evaluate long-term memory (Baarendse et al., 2008). In females, the number of electrical foot shocks in the learning trial was significantly higher in the METH-F1 group compared with the control-F1 group (Fig. 7A). In male mice, the retention latency in the memory trial was significantly lower in the METH-F1 compared with the control-F1 group (Fig. 7B). These results suggested that paternal METH exposure caused impairment of short-term memory in F1 females and impairment of long-term memory in F1 males. We previously reported that paternal MPH exposure reduced long-term memory in F1 males but did not affect memory in F1 females (Nakano et al., 2023). These findings indicated that MPH and METH have different effects on F1 females. Several studies reported the effects of METH on memory (Seminerio et al., 2013; Lee et al., 2011), although each study used male mice. This study determined sex differences in the effects of paternal METH exposure on the memory of F1 mice; further analysis of METH toxicity and sex differences is needed.
Metascape analysis of male F1 mice showed that GOs such as "behavior" and "neuron projection development" were enriched (Fig. 8). This suggested that paternal METH exposure affected behavior and neuron projection development of F1 mice. In addition, the GO for "behavior" included "learning or memory". Therefore, paternal METH exposure changed gene expression in male F1 mice, resulting in a memory decline. METH affects dopamine receptors and dopamine transporter (DAT) in mice (Shukla and Vincent, 2021), resulting in behavioral activation. In contrast, comprehensive analysis using RNA-seq showed no changes in the expression of Drd1, Drd2, and DAT, of which expressions were altered in the case of MPH, in F1 males. These were consistent with the results from spontaneous locomotor activity and acute METH treatment experiments (Figs. 4A and 5A). We performed RT-PCR on Gabrg2, Gabra5, and Cartpt, which are genes included in the GO of "behavior." GABRG2 is the γ2 subunit of the γ-aminobutyric acid type A (GABAA) receptor, and GABRA5 is the α5 subunit of the GABAA receptor, which are components of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the brain (Butler et al., 2018). CARTPT is a pre-propeptide of CART (Cocaine and amphetamine-regulated transcript) and its expression increases in the mouse striatum following the administration of cocaine and amphetamine (Piechota et al., 2012). Although no significant differences were observed, the expression levels of Cartpt, Gabrg2, and Gabra5 genes tended to be lower than in the control-F1 group (Fig. 9), consistent with the RNA-seq results. However, we could not identify genes that significantly fluctuated and strongly contributed to memory decline in the METH-F1 group. In addition, paternal MPH exposure resulted in changes in genes related to exocytosis that were reported to be involved in ADHD: Syt1 and Stx1a; however, paternal METH exposure did not affect these genes (Fig. 9).
The number of births at P1 and the survival rate at P4 in the METH-F2 group were comparable to those in the control-F2 group and with no significant differences (Table 2). Meanwhile, P4 body weight and the rate of weight gain to weaning (P21) were significantly higher in the METH-F2 group compared with the control F2 group, unlike F1 (Table 2, Fig. 10). The response differs across generations, although it was suggested that paternal METH exposure affects the feeding behavior and energy metabolism of descendants. Methamphetamine increases activity and causes anorexia in humans and rodents, leading to decreased food intake and weight loss (Bray, 1993; Liang et al., 2020). Additionally, grandchildren have significantly higher rates of obesity in humans if a grandfather was exposed to malnutrition (Costa, 2023). These results suggested that METH-treated sires (F0) could be mildly malnourished (Fig. S1), resulting in epigenetic changes and weight gain in METH-F2 mice.
The developmental tests showed that the METH-F2 group had a longer latency in the righting reflex test and lower scores in the wire-hanging maneuver compared with the control-F2 group (Fig. 11). However, it was thought that they had almost the same ability as the control-F2 group when comprehensively evaluated at approximately one week of age, including the results of the latency time of the wire-hanging maneuver and the cliff avoidance test. The developmental delay owing to paternal METH exposure observed in F1 mice was almost nonexistent in F2 mice. Evaluation of adolescent behavior showed that spontaneous locomotor activity was similar between the METH-F2 and control-F2 groups in males, whereas a significant decrease in spontaneous locomotor activity was observed in the female METH-F2 group (Fig. 12). Furthermore, locomotor activity after acute METH administration was significantly lower in the METH-F2 group (Fig. 13). These results suggest that paternal METH exposure decreases METH sensitivity in F2 mice, but does not affect METH sensitivity in F1 mice.
There were no significant differences between the two groups in each evaluation item of the EPM test in males (Fig. 14A). However, females in the METH-F2 group showed a significant increase in the time spent and distance traveled in the open arm, and a significant decrease in the number of entries into the closed arm (Fig 14B). These results suggested that paternal METH exposure does not affect anxiety-related behavior in F1 mice but decreases anxiety-related behavior in female F2 mice. In contrast, passive avoidance tests showed no significant difference between the METH-F2 and control-F2 groups in males or females (Fig. 15). This indicated that paternal METH exposure does not affect memory in F2 mice.
RNA-seq and Metascape analysis in the striatum of F2 males showed enrichment of GO terms such as “protein folding” and “protein processing in endoplasmic reticulum” (Fig. 16). GO such as “behavior” that were enriched in Metascape analysis of F1 males (Fig. 8) were not enriched in F2. In this study, the Metascape analysis was performed on male mice. However, behavioral changes were observed in females of F2 mice, so analysis of females will be necessary in the following study. This study could not clarify the mechanism by which paternal METH exposure caused changes in F1 and F2 growth and behavior. METH exposure damages sperm chromatin and DNA (Sabour et al., 2017). It is possible that the DNA of the damaged sperm was inherited and affected F1 and F2 mice. It will be necessary to conduct an epigenetic analysis of F0 and F1 mouse sperm DNA in the future.
In conclusion, this study demonstrated that paternal METH exposure resulted in delayed growth and decreased memory function in F1 mice, overweight in F2 mice, decreased METH sensitivity, and reduced anxiety-related behavior in female F2 mice. Changes that were not observed in F1 mice were often observed in F2 mice. This indicated that the effects of paternal METH exposure vary from generation to generation. The behavioral or gene expression changes in F1 seen with paternal administration of MPH were not observed with METH, indicating that similar stimulants have different effects on the next generation. The effects of abused drugs or medicines need to be examined in the long term in F1, F2, and beyond. Results of this study will contribute to education on abuse prevention among young generations by illustrating the potential for drug abuse to be a transgenerational hazard.
This work was supported by JSPS KAKENHI Grant No. 23K 27320. The authors are grateful to Dr. Toru Nishikawa (Department of Pharmacology, School of Medicine, Showa University) for the brain dissection, Dr. Fumihiro Ishikawa (Center for Biotechnology, Showa University) for the measurement of RNA Integrity Number, and Dr. Eisuke Inoue (Showa University Research Administration Center, Showa University) for the advice on statistical analysis.
Conflict of interestThe authors declare that there is no conflict of interest.