2024 年 49 巻 4 号 p. 151-161
2024 年 49 巻 4 号 p. 151-161
Alpha-glycosyl isoquercitrin (AGIQ) is composed of isoquercitrin and its glucosylated derivatives and has many biological activities, including anti-inflammatory, antioxidant, and anti-cancer properties. However, the effect of AGIQ administered orally on gut microbiota composition remains unclear. The objective of this study was to evaluate the effect of AGIQ on the gut microbiota of animals in different dose groups. Male rats and mice received different doses of AGIQ (1.5%, 3%, or 5% w/v) in diet for carcinogenic or chronic toxicity studies (rasH2 mice: 6 months; Sprague-Dawley rats: 12 months). Male minipigs received 100, 300, or 1000 mg/kg/day for 28 days. Fecal samples were collected from the different animal species and analyzed using 16S-rRNA gene sequencing. No significant changes were observed in alpha and beta diversity of the gut microbiota. Characteristic bacteria that responded to AGIQ were identified in each animal species, and, interestingly, Kineothrix alysoides, a butyrate-producing bacterium, was commonly detected in all three species, suggesting that it may be related to the biological activities of AGIQ. AGIQ selectively modulated the number of beneficial butyrate-producing commensal bacterium beneficial bacteria without changing the diversity of gut microbiota, which further supports the safe use of AGIQ in food products.
The mammalian gut microbiota, the largest and most complex micro-ecosystem of the organism, is known as “a hidden organ” (Wang et al., 2015). Many studies have shown that gut bacteria and their metabolites have a strong influence on metabolism (Tremaroli and Bäckhed, 2012), disease pathogenesis (Louis et al., 2014), and host immune function (Sjögren et al., 2009). The gut microbiota composition is shaped by multiple complex factors. Microbes in the gut are initially acquired from the mother during the birthing process (Dominguez-Bello et al., 2010) and then modified by environmental factors, and diet has a particularly important effect on the gut microbiota (Leeming et al., 2019). For instance, the gut microbiota composition of C57BL/6J mice was changed within one day after changing to a high-fat and high-sugar diet, and stabilized after seven days (Turnbaugh et al., 2009). Moreover, the reintroduction of a microbiota-accessible carbohydrate (MAC) diet failed to reverse the loss of gut microbial diversity caused by low-MAC diet over several generations (Sonnenburg et al., 2016). Finally, researchers found that the greatest changes in the gut microbiota composition occurred during two major socio-dietary shifts in human history by analyzing the oral microbiota in the skeletonized dental remains of people that lived in various eras (Adler et al., 2013). These findings showed the fast and dominant role of dietary habits in modifying the gut microbiota composition.
Quercetin (3,3',4′,5,7-pentahydroxyflavone) is a natural flavonoid that is widely found in fruits, vegetables, herbal medicines (Makino et al., 2009), and tea (Jeong et al., 2009). Moreover, quercetin exerts various beneficial effects, including antioxidant, anti-inflammatory, anti-allergic, and anti-cancer activities (Jeong et al., 2009). However, orally administered quercetin has low bioavailability because of its insolubility in water (Makino et al., 2009). Alpha-glycosyl isoquercitrin (AGIQ), also known as enzymatically modified isoquercitrin (EMIQ), is composed of isoquercitrin and its glucosylated derivatives and is produced via the enzymatic glycosylation of rutin (Formica and Regelson, 1995). It has been reported that the conjugation of quercetin with glucose could enhance its absorption in the small intestine due to the hydrophilicity of the sugar moieties (Makino et al., 2009). Moreover, AGIQ has been noticed as generally recognized as safe (GRAS) to the US Food and Drug Administration; it is recognized as a food additive by the Japan Food Sanitation Law (Japanese Ministry of Health and Welfare) and used in many products (Morita et al., 2011), ranging from beverages, wine coolers, and puddings to baked desserts, jams, and chewing gum (Hobbs et al., 2018).
Besides its food and beverage applications, AGIQ may exert many biological activities. For instance, AGIQ suppressed thioacetamide-induced preneoplastic liver cell lesions by its anti-inflammatory effect in rats (Fujii et al., 2013). AGIQ mitigated symptoms of Japanese cedar pollinosis by its antiallergic effect in subjects (Kawai et al., 2009). Moreover, AGIQ attenuated beta-naphthoflavone-induced hepatocellular tumor promotion through anti-inflammatory effect and alleviated oxidative stress in hepatocytes (Shimada et al., 2010). Although there are extensive data on the beneficial effects of AGIQ (Fujii et al., 2013; Hobbs et al., 2018; Morita et al., 2011), effects of AGIQ on the gut microbiota remain unclear.
In this study, relatively large doses of AGIQ were chosen to conduct a safety study in CByB6F1-Tg (HRAS)2Jic (rasH2) mice (Mahapatra et al., 2021), Sprague-Dawley (SD) rats (Nyska et al., 2016), and Göttingen minipigs (Maronpot et al., 2019), following good laboratory practice (GLP) guidelines. The rasH2 mouse is a transgenic strain that is useful for the study of genotoxic or non-genotoxic mechanisms (Eastmond et al., 2013; Usui et al., 2001). At present, the six-month study using rasH2 mice is considered the leading model to assess drug candidates (Eastmond et al., 2013; Usui et al., 2001). Therefore, the objective of this study was to evaluate AGIQ safety by investigating the effect of high doses of high-purity (> 97%) AGIQ on the gut microbiota composition in rasH2 mice, SD rats, and minipigs.
The rasH2 mice (7–9 weeks of age) and SD rats (5 weeks of age) were housed at 22 ± 2°C under a 14-hr light schedule (5:00 AM to 7:00 PM), and were offered a Purina Certified 5002 Meal Diet (Ralston Purina Co., St. Louis, MO, USA) and drinking distilled water ad libitum. The rasH2 mice and SD rats were acclimatized to the housing conditions for at least seven days before the experiments commenced. The rat and mouse studies, which were based on the Guide for the Care and Use of Laboratory Animals, were conducted in accordance with the Animal Welfare Act Regulations (9 CFR 1-4) and were approved by the Animal Care and Use Committee of Integrated Laboratory Systems, Inc. (Morrisville, NC, USA).
Pregnant Göttingen minipigs were acclimatized to the housing conditions for four weeks before the estimated due date. The experimental animals were the un-weaned piglets, housed in indoor pens containing infrared heat lamps at 15–24°C with a 12-hr light-dark cycle. The juvenile minipigs were fed by the sows on postnatal day (PND) 1 or 2. They were subsequently administered reconstituted milk supplement (Volac Faramate; Volac International Ltd., Devon, UK) every 3 hr at a dose of 150 g/L. The minipig study was approved by Envigo CRS Limited (Huntingdon, UK), according to the applicable sections of the United Kingdom Animals (Scientific Procedures) Act 1986 and the Amended Regulations of 2012.
Experimental designA 6-month carcinogenicity study was conducted on rasH2 mice. Forty male rasH2 mice were randomized into AGIQ-fed mice groups and a control group. The AGIQ-fed group animals were administered 1.5%, 3.0%, or 5.0% AGIQ (> 97% purity; San-Ei Gen F.F.I., Inc., Osaka, Japan) in diet (Mahapatra et al., 2021) for 6 months. A 12-month chronic toxicity study was conducted on SD rats. Forty male SD rats were randomized into three AGIQ-fed groups and a control group. The SD rats were administered 1.5%, 3.0%, or 5.0% AGIQ in diet for 12 months.
A four-week study was conducted on minipigs from PND 3 to PND 30; three AGIQ-fed groups with three animals in each group and a control group with two male animals. The different AGIQ-fed groups received 100, 300, or 1000 mg/kg/day AGIQ for 28 days, respectively, orally via reconstituted milk supplement administered via individual pan feeding. The AGIQ was administered in four daily oral doses (5 mL/kg body weight per dose). The doses used in this study were based on previously published studies (Maronpot et al., 2019). After the specified administration period, the animals were euthanized by CO2 asphyxiation. Cecal content (nominally 1.5 g) was collected from all animals, stored in an appropriate container, snap frozen over solid carbon dioxide, and subsequently stored at −80°C.
DNA isolation from cecal contentsMicrobial genomic DNA from the collected cecal contents was extracted using a QuickGene DNA tissue kit S (Kurabo, Osaka, Japan) according to the manufacturer’s instructions. Briefly, 40 mg of cecal contents, 15 mg of 0.2 mm glass beads (No.02, Toshin Riko Co., Ltd., Tokyo, Japan), and 200 µL of tissue lysis buffer were mixed and homogenized at 3,000 rpm for 120 sec using Micro Smash Cell Disrupter (MS-100, Tomy Seiko Co. Ltd., Tokyo, Japan). Proteinase K (25 µL) was added to the homogenized sample and incubated at 55°C for 1 hr. The sample was then centrifuged for 10 min at 14,000 rpm at 20°C and the supernatant was transferred to another microtube containing 180 µL of lysis buffer and incubated at 70°C for 10 min. After incubation, 240 µL of > 99% ethanol was added to the microtube and vortexed at the maximum speed for 15 sec. The whole lysate was transferred to the QuickGene Mini480 cartridge and pressurized. Wash buffer (750 µL) was applied to the cartridges three times. After the third wash, 200 µL of elution buffer was added and the mixture was incubated at 20°C. DNA was extracted after the third pressurization. The DNA concentration was adjusted to 20 ng/µL using elution buffer.
Library preparation and DNA sequencingThe 16S rRNA metagenome analysis was performed as previously described (Inoue et al., 2016). In brief, the V3-V4 region of the 16S rRNA gene was amplified using polymerase chain reaction (PCR; 95°C for 3 min, followed by 25 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, and a final extension at 72°C for 5 min) using primers 341F (5′-CCTACGGGNGGCWGCAG) and 805R (5′-GACTACHVGGGTATCTAATCC). The PCR product was verified using a Bioanalyzer DNA 1000 chip and the V3 and V4 primer pairs; the size of the Bioanalyzer trace after the Amplicon PCR step was ~550 bp. After purification of the PCR product, index PCR was performed on a thermal cycler using the following program: 9°C for 3 min, followed by 8 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, and a final extension at 72°C for 5 min. The amplicon of the index PCR was washed and the index PCR product was verified using reverse transcription-PCR with a primer pair (forward primer, 5′-AATGATACGGCGACCACCGAGAT; reverse primer, 5′-CAAGCAGAAGACGGCATACGA). The library concentration was determined by pooling each of the normalized amplicons using AMPure XP beads (Beckman Coulter Life Sciences, Indianapolis, IN, USA). Library quantification was performed using a library quantification kit. Sequence data were processed using Quantitative Insights into Microbial Ecology (QIIME) version 1.9 (http://qiime.org/) (Caporaso et al., 2010), USEARCH version 9.2.64 (https://drive5.com/usearch/) (Edgar, 2010), UCHIME version 4.2.40 (https://mybiosoftware.com/uchime-4-2-40-chimeric-sequences-detection.html) (Edgar et al., 2011), and VSEARCH version 2.4.3 (https://github.com/torognes/vsearch) (Rognes et al., 2016). The singletons genes were then removed. Operational taxonomic unit (OTU) assignment was conducted using the Ribosomal database project (RDP) classifier v2.10.2 with the Greengenes database (published in May 2013) (Balvočiūtė and Huson, 2017). Microbiome Analyst was used for the relative abundance of taxa analysis (https://www.microbiomeanalyst.ca/) (Chong et al., 2020). All 16S rRNA sequencing data have been deposited and will be available under the accession number PRJNA856077 on Jan/2023.
Fecal cultureCecal contents were collected from 5-week-old male C57BL/6 mice and made the suspension by mixing with trypticase soya broth + 5% [vol/vol] bovine serum albumin (100 mg cecal contents + 10 mL medium). 1 mL of fecal suspension with 0, 0.15%, 0.3% or 0.5% AGIQ (final concentration) was cultured in glass vessels with rubber stoppers containing 10 mL medium in advance. Gassing of media and cultures with oxygen-free gas to create anaerobic conditions. After incubation under 37°C for 24 hr, the suspension was collected and homogenized at 3,000 rpm for 5 min to extract DNA.
Quantitative real-time polymerase chain reaction (qRT-PCR)Fecal DNA was adjusted to 20 ng/µL in order to carry out qRT-PCR for Kineothrix alysoides with a total reaction volume of 10 µL using the PowerTrack™ SYBR Green master mix (Thermo Fisher Scientific, Waltham, MA, USA). The expression level of K. alysoides relative to Total-3 was determined using the 2−ΔΔCT method. The primers used were as follows: Total-3 (5′-ACT CCT ACG GGA GGC AGC AGT-3′ and 5′-GTA TTA CCG CGG CTG CTG GCA C-3′) and K. alysoides (5′- GGA AGA AAG CCA TGA CGG TA-3′ and 5′-TCG AGC CTC AAC GTC AGT TA-3′).
Statistical analysisMicrobiome Analyst was used for data processing and microbiome analyses (https://www.microbiomeanalyst.ca/). Rarefaction curves were construct to assess species richness and estimate the sequencing depth. Moreover, alpha diversity was compared using Chao1, ACE, Shannon and Simpson indexes by the ANOVA test. Beta diversity was detected using principal coordinate analysis (PCoA) on the Bray-Curtis distance and permutational multivariate analysis of variance (PERMANOVA). Differentially abundant bacterial taxa were showed by Linear discriminant analysis (LDA) effect size (LEfSe) using MicrobiomeAnalyst. Log LDA score was set to 4.0 and p-value < 0.05. The levels of K. alysoides in cecal contents after AGIQ incubation in vitro was compared using one-way ANOVA by GraphPad Prism (version 6.01). Statistical significance was set at * p < 0.05, ** p < 0.01.
To evaluate whether AGIQ affected bacterial richness, the microbiota composition was analyzed in rasH2 mice. The rarefaction curve (Fig. 1A) showed that species richness no longer increased as the amount of data extracted increases, indicating that the sequencing depth was reasonable. We then measured bacterial community richness in terms of alpha diversity after AGIQ administration using the Chao1, ACE, Shannon, and Simpson indexes, which describes the number of OTUs. The bacterial species richness was similar between the control and AGIQ groups in rasH2 (Fig. 1B). Beta diversity is an important part of biodiversity and describes the level of overlap in richness by measuring the shared degree of membership or structure between communities. Herein, PCoA indicated that the beta diversity did not differ between the control and AGIQ groups in rasH2 mice (p < 0.766) (Fig. 1C).

Effect of alpha-glucosyl isoquercitrin (AGIQ) on gut microbiota in rasH2 mice. (A) Rarefaction curves of species richness showing the sequencing depth, (B) boxplots of alpha diversity indices, namely Chao1, ACE, Shannon and Simpson for bacterial, (C) beta diversity, and (D) top 15 features ranked by linear discriminant analysis (LDA) score (p < 0.05) with percent identity > 90% (left panel), and log-transformed counts of Mucispirillum schaedleri and Marasmitruncus massiliensis in the feces of mice treated with 0% (red) and 1.5% (green), 3% (blue), and 5% AGIQ (purple), respectively (right histograms). Log-transformed counts were converted with log base 2 using the abundance. The rarefaction curves for each sample were displayed in different colors.
To determine the specific bacterial taxa related to AGIQ, LEfSe method was used to analyze the fecal microbiota community of the control and AGIQ groups in mice. The top 15 features (p < 0.05) ranked by LDA score > 2 are shown, including Mucispirillum schaedleri, Marasmitruncus massiliensis, K. alysoides, Lachnoclostridium edouardi, Stomatobaculum longum, Anaerotignum lactatifermentans, L. edouardi, Enterorhabdus mucosicola, Acetatifactor muris, Coprococcus phoceensis, one overlapped species, and three unidentified features (Fig. 1D). In particular, levels of M. schaedleri and M. massiliensis in the control and AGIQ groups are shown. 5% AGIQ administration increased the level of M. schaedleri. AGIQ (1.5%) increased the level of M. massiliensis; however, 3% and 5% AGIQ caused a decrease.
Changes of the gut microbiota composition in AGIQ-treated SD ratsTo evaluate the safety of AGIQ on the gut microbiota, the microbiota composition was explored in SD rats. As shown in Fig. 2A, rarefaction curves of the majority of the samples analyzed reached saturation. The alpha diversity was then estimated on the basis of indexes: Chao1, ACE, Shannon and Simpson index (Fig. 2B). Chao1 and ACE indexes were lower in the 5% AGIQ group than in the 1.5% AGIQ group and 1.5% and 3% AGIQ groups, respectively, while there was no significant difference in these indexes between the control group and AGIQ groups. Beta diversity analysis revealed that the community composition of the gut microbiome was affected by AGIQ administration (Fig. 2C; p < 0.03).

Effect of alpha-glucosyl isoquercitrin (AGIQ) on gut microbiota in Sprague-Dawley rats. (A) Rarefaction curves of species richness showing the sequencing depth, (B) boxplots of alpha diversity indices, namely Chao1, ACE, Shannon and Simpson for bacterial, (C) beta diversity, and (D) top 15 features ranked by linear discriminant analysis (LDA) score (p < 0.05) with percent identity > 90%, and log-transformed counts of Lacrimispora sphenoides and Oscillibacter ruminantium in the feces of rats treated with 0% (red), 1.5% (green), 3% (blue), and 5% AGIQ (purple) AGIQ, respectively. Log-transformed counts were converted with log base 2 using the abundance. The rarefaction curves for each sample were displayed by different colors. * p < 0.05, ** p < 0.01.
LEfSe analysis identified the top 15 bacterial taxa (ranked by LDA score) with differences in relative abundance between the control group and AGIQ groups, including Lacrimispora sphenoides, Oscillibacter ruminantium, Ruminococcus albus, Anaerostipes caccae, Lawsonibacter asaccharolyticus, Clostridium cuniculi, Desulfovibrio eutactus, Coprococcus eutactus, Ruminococcus champanellensis, Clostridium indolis, Allobaculum stercoricanis, Streptomyces griseocarneus, Monoglobus pectinilyticus, and two unidentified features (Fig. 2D). Moreover, the level of L. sphenoides was decreased by three AGIQ groups compared with the control group in feces. 1.5% AGIQ reduced the level of O. ruminantium; however, the level of O. ruminantium was higher in the 3% and 5% AGIQ groups than in the control group.
Effects of AGIQ on the gut microbiota composition in minipigsTo explore the effect of AGIQ in the gut microbiota composition in minipigs, the microbiota composition was explored in minipigs. As shown in Fig. 3A, rarefaction curves of the majority of the samples analyzed reached saturation. We employed alpha diversity to investigate total richness by Chao1, ACE, Shannon and Simpson indexes in minipigs. The Shannon index was lower in the 1000 mg/kg AGIQ group than those in the 100 and 300 mg/kg AGIQ groups, while there was no significant difference in alpha diversity between the control group and AGIQ groups (Fig. 3B). Furthermore, the PCoA indicated that there were no significant differences in beta diversity of the gut microbiota between the control and AGIQ groups (p < 0.405) (Fig. 3C).

Effect of alpha-glucosyl isoquercitrin (AGIQ) on gut microbiota in minipigs. (A) Rarefaction curves of species richness showing the sequencing depth, (B) alpha diversity indices, namely Chao1, ACE, Shannon and Simpson for bacterial, (C) beta diversity, and (D) top 14 features ranked by linear discriminant analysis (LDA) score (p < 0.05) with percent identity > 90%, and log-transformed counts of Kineothrix alysoides and Clostridium hylemonae in the feces of pigs treated with 0 (red), 100 (green), 300 (blue), and 1000 (purple) mg/kg/day AGIQ, respectively. Log-transformed counts were converted with log base 2 using the abundance. * p < 0.05, ** p < 0.01.
14 features with differences in relative abundance between the control group and AGIQ groups ranked by LDA score were shown based on LEfSe analysis, including K. alysoides, Clostridium hylemonae, Prevotella stercorea, S. griseocarneus, Butyricimonas faecalis, Faecalibacterium prausnitzii, Neglecta timonensis, Desulfovibrio piger, N. timonensis, Odoribacter splanchnicus, S. griseocarneus, and three unidentified features (Fig. 3D). Moreover, 100, 300, and 1000 mg/kg AGIQ administration significantly increased the level of K. alysoides and decreased the level of C. hylemonae compared with the control group.
Shared OTUs that were significantly different between control and AGIQ groups among rats, mice, and minipigsTo determine the specific bacterial taxa related to AGIQ, the LEfSe method was used to analyze the fecal microbiota community of the control and AGIQ groups in the three different kinds of experimental animals. To clarify the overlapping of the microbial species, we converted bar plots into a Venn diagram to show the shared OTUs among the animals. Based on LEfSe comparisons, the number of species with differences in relative abundance between the control and AGIQ groups in rasH2 mice, SD rats, and minipigs, were 11, 48, and 11, respectively (Fig. 4A). The results showed that only one OTU (9.1%) was shared between rasH2 mice and minipigs. This species, K. alysoides, was more abundant in the AGIQ groups compared with the control in rasH2 mice and minipigs. Four shared species were identified between rasH2 mice and SD rats, accounting for 36.4% and 8.3% of the species in the corresponding animals, respectively. These species were K. alysoides, L. edouardi, E. mucosicola, and A. muris. Among them, the abundance of K. alysoides and E. mucosicola was higher in the AGIQ groups than in the control group, and L. edouardi was less abundant in AGIQ groups than in the control group in both rasH2 mice and SD rats. However, except for the 3% and 5% AGIQ groups in rasH2 mice, AGIQ administration in SD rats and 1.5% AGIQ administration in rasH2 mice increased the level of A. muris compared with the control group. There were three overlapping OTUs between SD rats and minipigs, that is, 6.3% and 27.3% of the species were present in SD rats and minipigs, respectively. These OTUs were K. alysoides, N. timonensis, and S. griseocarneus. K. alysoides and S. griseocarneus were significantly more abundant in AGIQ-fed groups than in the control group, whereas AGIQ administration decreased the level of N. timonensis in both SD rats and minipigs.
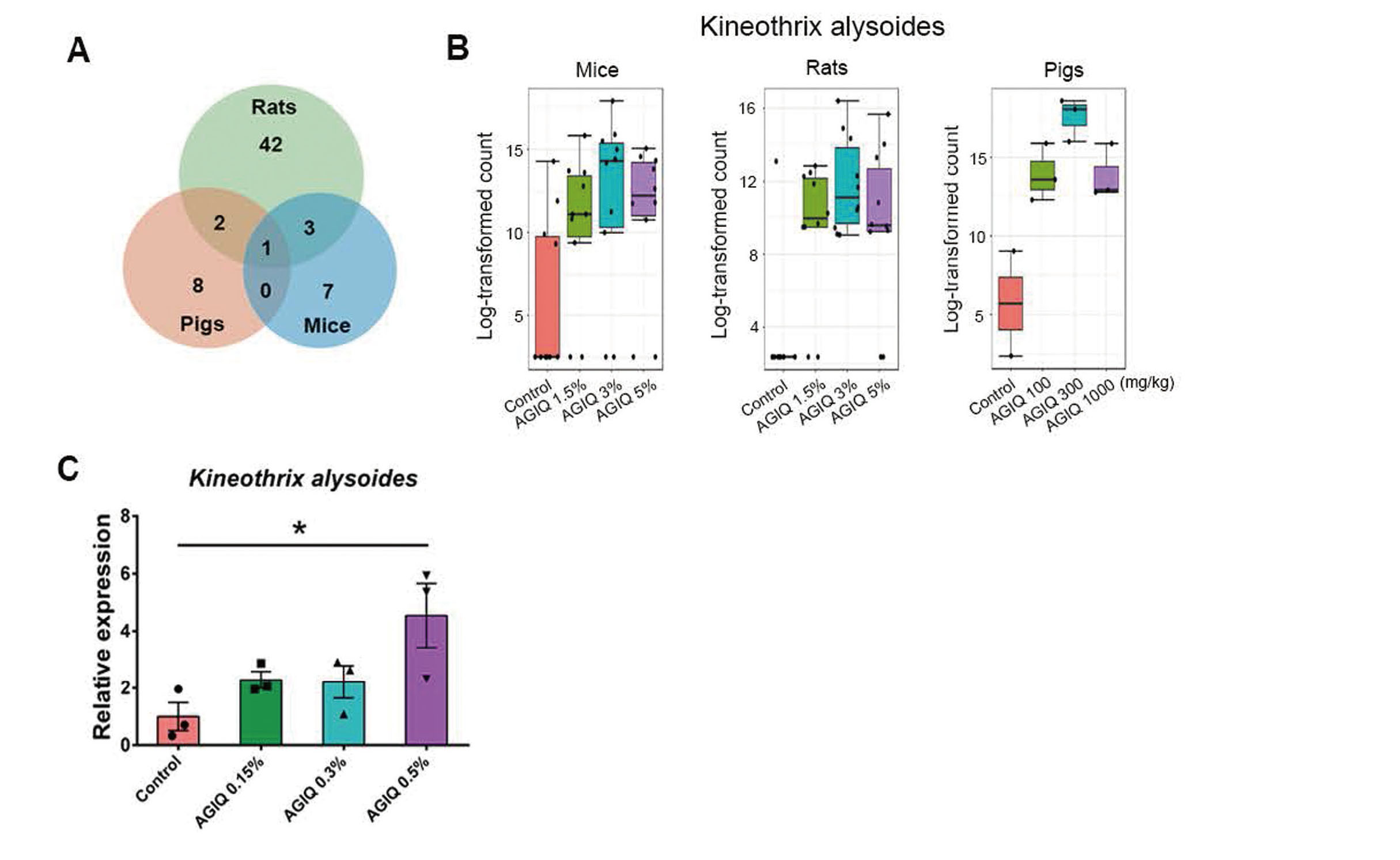
Effect of Alpha-glucosyl isoquercitrin (AGIQ) on the abundance of Kineothrix alysoides. (A) Venn diagram showing the overlap of operational taxonomic units (OTUs) with significant differences between the control and alpha-glucosyl isoquercitrin (AGIQ)-fed groups in fecal microbiota among the three groups. (B) Comparison of counts of Kineothrix alysoides between control groups and AGIQ groups in fecal samples from mice, rats, and pigs. Log-transformed counts were converted with log base 2 using the abundance. (C) The effect of AGIQ on the abundance of K. alysoides after mouse fecal culture in vitro using qRT-PCR. * p < 0.05.
The Venn diagram (Fig. 4A) shows that only one OTU (K. alysoides) with a total richness of 63 overlapped among all animals, that is, 9.1%, 2.1%, and 9.1% of OTUs were present in rasH2 mice, SD rats, and minipigs, respectively. K. alysoides was significantly more abundant in the fecal microbiota of AGIQ groups than in control samples in rasH2 mice, SD rats, and minipigs (Fig. 4B).
To further detect the effect of AGIQ on the level of K. alysoides, mouse cecal contents were cultured with 0, 0.15%, 0.3%, or 0.5% AGIQ, respectively, for 24 hr to detect the level of K. alysoides using qRT-PCR. As shown in Fig. 4C, 0.5% AGIQ incubation could significantly increase the level of K. alysoides compared with the control group. This result further showed the promoting effect of AGIQ on the abundance of K. alysoides in vitro.
A strong link between disturbances in the gut microbiota and several diseases is well known. In most case, lower richness and diversity of gut microbiota was seen in patients compared to that of healthy individuals. Thus, the research on foods and compounds that affect the gut microbiota composition and diversity remains important. AGIQ, a polyphenol, has been shown to have a higher intestinal absorption rate and bioavailability than quercetin and isoquercitrin because of the conjugated glucose moieties (Hobbs et al., 2018). Researchers have assessed the genotoxicity and toxicity of cyclodextrin glucosyltransferase, which is an enzyme capable of producing AGIQ via rutin glycosylation and results demonstrated its safety (Hobbs et al., 2018). Moreover, a 90-day toxicity assessment of AGIQ in SD rats was performed to show its safety (Nyska et al., 2016). However, there is still a critical information gap regarding the comprehensive effect of AGIQ on the gut microbiota. Consequently, this study aimed to explore the differences of the gut microbiota in rasH2 mice, SD rats, and minipigs after AGIQ administration.
Polyphenols, which are bioactive non-nutrient plant compounds, may influence intestinal ecology (Laparra and Sanz, 2010). In the present study, the Chao 1 and ACE indexes were lower in the 5% AGIQ group than in the 1.5% AGIQ group and 1.5% and 3% groups in rats, respectively, and the Shannon index was lower in the 1000 mg/kg AGIQ group than those in the 100 and 300 mg/kg AGIQ-fed groups in minipigs. However, there was no significant difference in these indexes between control groups and AGIQ groups in rasH2 mice, SD rats and minipigs, indicating no difference in alpha diversity after AGIQ administration compared with the control group. And AGIQ affected beta diversity in SD rats (p < 0.03), whereas AGIQ administration had no effect on beta diversity in rasH2 mice and minipigs. These findings suggest that the alpha and beta diversity of the gut microbiota is largely not influenced by AGIQ. Polyphenols and their metabolic products may selectively inhibit pathogen growth while upregulating the growth of beneficial bacteria, affecting the gut microbiota composition (Lee et al., 2006). Polyphenols from tea have been shown to inhibit the growth of Bacteroides spp., Clostridium spp., and Escherichia coli (Lee et al., 2006), while resveratrol has also been shown to increase the counts of beneficial bacteria (Bifidobacterium spp. and Lactobacillus spp.) (Larrosa et al., 2009). Another study showed that anthocyanins from berries inhibited the counts of pathogenic Staphylococcus spp., Salmonella spp., Helicobacter pylori, and Bacillus cereus (Puupponen-Pimiä et al., 2005). Similarly, in the present study, AGIQ administration stimulated the counts of beneficial butyrate-producing commensal bacterium K. alysoides and the levels of opportunistic pathogen M. schaedleri and O. ruminantium (Song et al., 2023; Sydenham et al., 2014) was increased in high dose AGIQ (5%) group in rats and minipigs, respectively, whereas AGIQ inhibited the count of C. hylemonae. About M. schaedleri, some previous studies have shown that it could link to inflammatory bowel disease (Herp et al., 2021), high-fat diet (Ussar et al., 2015), stress (Jašarević et al., 2017) and diseases like Rheumatoid Arthritis (Xiao et al., 2018) or Parkinson’s Disease (Lin et al., 2019). However, increasing evidence indicates that the same bacterial strain termed as “pathobiont” could also exert health-promoting functions on its host in a different scenario (Herp et al., 2021). M. schaedleri may exert a beneficial role for application against human Salmonella infection (Herp et al., 2019). About O. ruminantium, it is probable that the related report is rare. And there were 4 cases of O. ruminantium bacteremia from hospitals across Denmark from 2001 to 2010 (Sydenham et al., 2014). Although AGIQ administration induced changes in the counts of several strains of bacteria, the alpha and beta diversity of the gut microbiota was not influenced by AGIQ except for beta diversity between different groups in rats.
Short-chain fatty acids (SCFAs), including acetate, butyrate, and propionate generated by intestinal bacteria, have been shown to play an important role in intestinal health by protecting the intestinal mucosal barrier, anti-inflammatory effect, and positive effects on enterocyte growth and differentiation (Xiong et al., 2020, Kles and Chang, 2006, Hamer et al., 2008). A decrease in the production of SCFAs can increase the incidence of colorectal cancer (Ganapathy et al., 2013). Dietary intake of polyphenols, such as consuming tea, could promote the growth of specific bacteria in the gut, resulting in intestinal pH changes, which increase the production of SCFAs leading to obesity prevention in a mouse model (Qiao et al., 2020). In addition, continuous administration of a polyphenol-rich black tea extract induced a high level of SCFAs in an in vitro gastrointestinal model (van Dorsten et al., 2012). In this study, among the 63 differentially abundant gut microbiota species, only one OTU was identified as being shared among all samples, namely K. alysoides, which produces butyrate. AGIQ administration significantly upregulated the count of K. alysoides in rasH2 mice, SD rats, and minipigs. Moreover, in vitro experiment further proved this effect. These findings showed that AGIQ is a safe food additive that selectively modulates the number of beneficial bacteria without changing the diversity of gut microbiota in rasH2 mice, SD rats, and minipigs.
However, the most important limitation of this research is the sample size in minipigs. Although sample size is 10 in rasH2 mice and SD rats, due to practical constraints, only 2 and 3 valid sample size in control and each AGIQ-fed group has been reached in minipigs. Nevertheless, consistent results showing the alpha and beta diversity of the gut microbiota was largely not influenced by AGIQ and AGIQ may selectively increase the counts of beneficial bacteria were obtained in three kinds of animals.
In summary, the present study reported that AGIQ largely did not change gut microbial diversity in rasH2 mice, SD rats, and minipigs. Moreover, AGIQ may selectively increase the counts of beneficial bacteria in the cecal contents. The comprehensive evaluation here supplemented existing data on the effect of AGIQ on gut microbiota, which supports the safe use of AGIQ in food and beverages.
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interestThe authors declare that there is no conflict of interest.