2021 年 70 巻 2 号 p. 35-43
2021 年 70 巻 2 号 p. 35-43
The year 2020 will be remembered for the coronavirus disease 2019 (COVID-19) pandemic, which continues to affect the whole world. Early and accurate identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is fundamental to combat the disease. Among the current diagnostic tests, real-time reverse transcriptase–polymerase chain reaction (RT-qPCR) is the most reliable and frequently used method. Herein, we discuss the interpretation of RT-qPCR results relative to viral infectivity. Although nasopharyngeal swab samples are often used for RT-qPCR testing, they require collection by trained medical staff. Saliva samples are emerging as an inexpensive and efficient alternative for large-scale screening. Pooled-sample testing of saliva has been applied for mass screening of SARS-CoV-2 infection. Current policies recommend isolating people with borderline cycle threshold (Ct) values (35<Ct <40), despite these Ct values indicating minimal infection risk. We propose the new concept of a “social cut-off” Ct value and risk stratification based on the correlation of Ct with infectivity. We also describe the experience of RT-qPCR screening of saliva samples at our institution. It is important to implement a scientific approach to minimize viral transmission while allowing economic and social activities to continue.
The unprecedented turmoil caused by the coronavirus disease 2019 (COVID-19) pandemic continues worldwide, although the rapid development of vaccines1 has started to give hope for recovering normalcy in the near future. Globally, as of December 31, 2020, over 83.8 million people were infected, and approximately 1.8 million people died from this disease.2 In trying to curb the surge of infections, countries around the world have imposed social restriction measures, such as limiting travel, closing businesses and schools, banning large group events, and mandatory quarantines. The resultant impact on people’s lives has been enormous, including unemployment, worsened food insecurity, increased incidents of domestic violence, and diverse effects on social and individual psychological well-being.3,4,5 To alleviate social and economic damage while prioritizing public health, it is imperative to detect infection early and minimize further spread of the virus.
In Japan, the first case of COVID-19 was reported on January 16, 2020.6 Besides the outbreak on the Diamond Princess cruise ship in February,7 Japan had its first wave from late March to April, during which a state of emergency was declared. The Japanese government requested schools and businesses to close, asked individuals to refrain from traveling and commuting, and encouraged remote learning and working. The state of emergency was lifted on May 25, 2020, after the number of daily infections was brought under control. The easing of restrictions resulted in a second wave between July and August, which was brought under control in September. At the time this manuscript was written (December 2020–January 2021), Japan was facing a third wave, during which the highest number of daily new confirmed and severe cases were reported since the beginning of the pandemic (Fig. 1A). As of December 31, 2020, the total number of patients diagnosed with COVID-19 surpassed 230,304, and 3459 people have died of COVID-19 in Japan.6 Similar to the nationwide trend, Tokyo has had three waves of infections; it also had the highest number of infections and deaths in the country (Fig. 1B).8
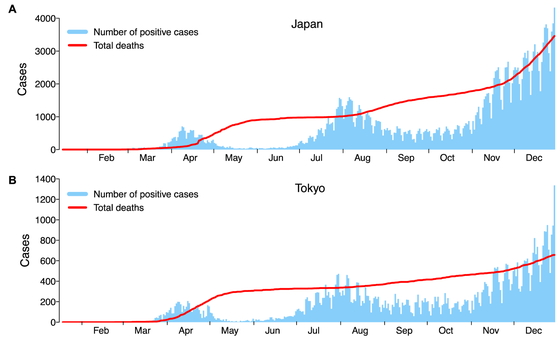
The number of daily reported PCR-positive cases and total deaths in 2020 (A) in Japan and (B) in Tokyo. The data for Japan were obtained from https://www.mhlw.go.jp/stf/covid-19/open-data_english.html; the data for Tokyo were obtained from https://stopcovid19.metro.tokyo.lg.jp/en.
COVID-19 is caused by infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There are two main analytical methods to detect SARS-CoV-2 infection: direct viral detection tests and antibody tests to assess current, recent, or previous infections.3,9 Direct viral detection tests are able to detect SARS-CoV-2 nucleic acid or protein antigens using samples from the respiratory system (such as nasal or oral swabs, or saliva) to indicate infection with SARS-CoV-2. Therefore, direct viral detection tests are recommended to diagnose acute and current infections in both symptomatic and asymptomatic individuals to guide contact tracing and determine isolation measures.
The gold standard method for detecting SARS-CoV-2 viral RNA is real-time reverse transcriptase–polymerase chain reaction (RT-qPCR).10 To date, different genes within the virus have been used as targets, including the envelope (E), spike (S), nucleocapsid (N), and open reading frame (ORF)1ab genes, with the N gene being the most prevalently used.3 Antigen testing is another approach that detects the presence of SARS-CoV-2 viral antigens. The most commonly used antigens in SARS-CoV-2 antigen tests are the N and S proteins.3 Although antigen tests have the benefit of a quick turnaround time (less than 30 min), their sensitivity is significantly lower than that of RT-qPCR.11,12 In this context, RT-qPCR techniques have higher sensitivity and specificity than antigen tests do and, therefore, can minimize the risk of misidentifying patients having the potential to transmit the virus.13,14,15
While direct viral detection tests can identify acutely infected patients, antibody tests can be positive during the acute infection and after the resolution of the infection. Samples for antibody tests are typically collected from the blood. Antibody tests for SARS-CoV-2 infection are useful tools for surveillance and epidemiologic studies. Recently, the U.S. Food and Drug Administration (FDA) authorized the first test to detect neutralizing antibodies that have been shown to decrease in vitro SARS-CoV-2 infection.16 Since COVID-19 is an emerging infectious disease, it remains to be elucidated whether detecting neutralizing antibodies in an infected person is associated with protective immunity against this virus.
For diagnostic RT-qPCR testing of SARS-CoV-2, samples are obtained from the respiratory system, such as the nasopharynx, oral cavity, or saliva.10 Although nasopharyngeal swab samples have been most frequently used for RT-qPCR testing, such samples require collection by trained medical staff, pose infection risks, and utilize both personnel and personal protective equipment (PPE).17 In contrast, saliva samples can be collected by non-experts and even allow for self-collection. Several studies have compared matched nasopharyngeal swabs and saliva samples obtained from patients with PCR-confirmed or clinically suspected SARS-CoV-2 infection. There was a high concordance observed between the RT-qPCR cycle threshold (Ct) values of matched nasopharyngeal and saliva samples obtained early (within 9 days from symptom onset) in the course of the disease.18,19,20,21
Uwamino et al. also assessed the stability of saliva samples and demonstrated that RT-qPCR Ct values were not affected even after storage at room temperature for more than 7 days.18 Interestingly, the copy number of SARS-CoV-2 RNA was higher in saliva than that in nasopharyngeal swabs from hospitalized COVID-19 patients.22 Because SARS-CoV-2 can be detected in the saliva of asymptomatic individuals,20,22 saliva has been proposed as a non-invasive alternative to nasopharyngeal swabs to facilitate large-scale RT-qPCR screening without posing a risk to healthcare workers.
In principle, a PCR result is considered “positive” when the Ct value is less than 40.10 However, there has been debate over the optimal cutoff for the diagnosis of COVID-19 because suggested thresholds vary between countries and experts.23 For example, some groups used a cut-off Ct value <38 for the diagnosis of SARS-CoV-2,22,24 whereas other groups used a lower cut-off value.25 It is important to note that Ct value cutoffs are established by manufacturers through evaluation of known positive and negative samples; consequently, not all RT-qPCR tests use the same Ct cutoff value.
Multiple studies have indicated that RT-qPCR can detect non-viable viral RNA fragments and viral copy numbers that are lower than those observed during infection.26,27 Using a cut-off Ct value <40 might misidentify individuals who are not infectious and lead to unnecessary isolation. To maintain social and economic activities while preventing the spread of infection, it is crucial to have guidelines that are reasonable and realistic.
Several groups have examined the correlation between RT-qPCR Ct values and infectivity,26,28,29,30,31 suggesting that much lower Ct values could be used for practical quarantine control methods. For example, a group from France performed serial RT-qPCR testing and compared Ct values with infectivity in a cell culture model using nasopharyngeal samples from 155 RT-qPCR-confirmed COVID-19 patients.26 The authors demonstrated that the success rate of viral culture decreased progressively as Ct values increased. They also observed that the virus was not isolated from samples collected later in the course of disease (8 days after symptom onset), whereas viral RNA was still detected by RT-qPCR. They concluded that patients with Ct values of at least 34 no longer pose a threat of viral transmission.26 Another study from Canada that examined the correlation between RT-qPCR Ct values and virus isolation in cell culture showed that infectivity (as defined by growth in cell culture) is dependent on both Ct values (less than 24) and the stage of the disease (less than 8 days from symptom onset).28 A strong relationship between Ct values and virus isolation has also been reported by a study group in the U.K. The authors showed that there was no statistically significant difference in Ct values between those with asymptomatic, mild–moderate, and severe symptoms, and that samples from asymptomatic and symptomatic patients exhibited similar virus isolation results, based on the Ct values and time points in their disease course.29 Another study from the U.S. showed that virus growth in cell culture was highly efficient in samples with Ct values of 10–20 (76.7% positive isolation rate), and dropped to 24.1% for Ct values of 20–30 and to 2.9% for Ct values of 30–40. There was one outlier case of positive viral culture in a sample with a Ct value above 30 (Ct =32.1), but the rest of the 47 culture-positive samples had Ct values lower than 30, with a mean Ct value of 18.8.31 Further, Yamayoshi et al. reported that no virus isolation was observed in samples with Ct values higher than 30.14
Although there are variabilities of Ct value thresholds for viral infectivity in cell culture models, with Ct values of 34, 30, and 24 being indicated, the common takeaway interpretation from these studies is that the viral load at the end of the RT-qPCR cycle (>34 Ct) may not reflect virus replication capacity. When we applied the formula (10^((Ct–43.023)/–3.718))*75*(1000/300) as previously described,22 a Ct value of 34 indicates 1 × 106 viral particles in 10 mL of nasopharyngeal or saliva samples (Fig. 2). Given that normal social activity does not involve exchanging such amounts of saliva or nasal discharge, the risk of transmission is very low. However, the amount of virus needed for person-to-person transmission of SARS-CoV-2 is still unknown.
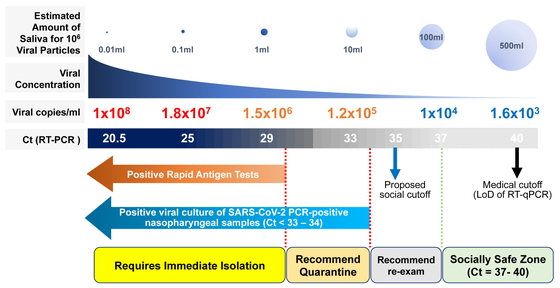
Proposed risk stratification based on RT-qPCR Ct values and rapid antigen testing. Viral copies/ml were estimated from RT-qPCR Ct values using the formula provided by Wyllie et al.22 The estimated amount of saliva required to contain 106 viral copies and the corresponding viral concentrations are shown. Data for positive viral cultures of SARS-CoV-2 PCR-positive nasopharyngeal samples (Ct <33–34) are from La Scola et al.26 and Singanayagam et al.29 A new “social cut-off” at Ct values >35 is proposed based on these two and other studies showing the correlation between Ct values and viral culture recovery rates,26,28,29,30,31 thereby allowing individuals to continue their social activities with follow-up testing recommended. The limit of detection (LoD) is generally considered to be the lowest concentration of target that can be detected in ≥95% of repeat measurements. Note that LoDs of currently approved RT-qPCR kits for SARS-CoV-2 vary significantly,25,32 and the medical cut-off shown in the figure should be interpreted with caution. Ct, cycle threshold.
From the findings above, we propose using the RT-qPCR Ct values to stratify the risk of viral transmission as follows (Fig. 2):
– Patients with Ct values <30 are considered highly infectious, requiring immediate isolation and contact tracing. The tested individual should be reported to the local health center and should seek advice on medical treatment.
– Patients with Ct values between 30 and 34 are considered moderately infectious. The tested individual should refrain from social gatherings and quarantine themselves.
– Patients with Ct values between 34 and 37 are considered as belonging to a “gray zone,” allowing the tested individual to continue social activity but with adequate standard precautions33 (such as hand hygiene, PPE, respiratory etiquette, cleaning, and disinfection), physical distancing, and contact tracing. As implied by the word “gray,” individuals are recommended to have a follow-up test within the next few days and to closely monitor themselves for symptoms. We propose the new concept of a “social cut-off” at a Ct value <35, based on studies showing the correlation between Ct values and viral culture recovery rate.26,29,31
– Patients with Ct values >37 are considered non-infectious, allowing the tested individual to continue social activity with standard precautions.33 One must keep in mind that a Ct >37 (a “negative test”) does not completely rule out the presence of the virus, because there is a risk of early infection with low viral load. Decisions on social activity levels for a tested individual should be based not only on the Ct values but also on the combination of clinical observation and patient history, including recent exposure. It would be safer to follow up these individuals, especially those in high-risk environments such as hospitals, nursing homes, and schools to prevent unaddressed cluster outbreaks.
Rapid qualitative antigen testing has been widely implemented because it is easy, quick, and inexpensive. In general, rapid antigen tests detect patients whose RT-qPCR Ct values are below 30.13,14,34 When RT-qPCR is not easily accessible, rapid antigen testing should be considered. Individuals with a positive antigen test result must be advised to immediately isolate themselves and should be provided with medical care if they develop any symptoms.
Limitations of diagnosing COVID-19 using RT-qPCRThe RT-qPCR method has several limitations. First, a test may return a false-negative result,35 as experienced with other test modalities. To ensure both a safe and active society during a pandemic, it would be ideal to follow up individuals who were exposed to the virus or have suspicious symptoms and to encourage routine testing rather than snapshot, one-time testing. Second, different probes and primer targets result in different amplification efficiencies and Ct values.36,37 It is known that PCR amplification efficiencies critically influence the Ct values; for example, the number of viral genome copies estimated by a Ct value of 37 in PCR with an amplification efficiency of 100% is equal to that estimated by a Ct value of about 40 in PCR with an amplification efficiency of 90%. Each local laboratory in charge of diagnostic RT-qPCR testing should be aware of these differences when optimizing standard operating procedures and of the importance of regular laboratory quality checks and controls. Third, RT-qPCR can result in cross-contamination, sample carry-over, and false-positive results.38,39 Steps for monitoring and reducing contamination have been proposed.39 Regular quality measurements are essential when using RT-qPCR results for risk stratification. Fourth, mutations in the viral genome may compromise the sensitivity of RT-qPCR testing to detect SARS-CoV-2, as has been demonstrated in some reports.40,41 Consequently, it is critical to monitor the genetic evolution of this virus for the emergence of mutations that might negatively affect the accuracy of RT-qPCR-based diagnosis and to use more than two regions as targets to avoid false-negative results. Comparing the sensitivity and specificity of several commercially available PCR kits for detecting variants prevalent in each region will also minimize the risk of false-negative results. Finally, a new variant identified in the U.K. that is spreading across several other countries has renewed worldwide concern because it has been revealed to be more virulent than previous circulating viruses.42,43 If this lineage (known as B.1.1.7 and characterized by a range of 14 non-synonymous mutations and 3 deletions) becomes the dominant strain in the region, the social cut-off proposed here may have to be adjusted.
The effectiveness of sample pooling for RT-qPCR has been validated for other infectious diseases, including syphilis, malaria, HIV, and influenza.44,45 With the rising demand for large-scale testing for SARS-CoV-2, especially for screening purposes, pooled-sample testing offers a promising strategy to enable large-scale screening and save supplies, time, and human resources.45 Sample pooling is performed by mixing different samples and performing the test on this “pool” as if it were a single sample. If the result is negative, each sample in the pool is considered negative and no further individual testing is required. If the pool tests positive, then individual testing is required. Therefore, pooled sample testing can increase the testing capacity.45 Multiple groups have evaluated the effectiveness and accuracy of sample pooling strategies for detecting SARS-CoV-2. When considering the sensitivity and specificity of the test results, the optimal pool size (the number of samples in a pool) varies depending on the prevalence or positivity rate in the community. For example, a pool size of around 30 has been shown to be effective in low-prevalence areas.44 The dilution of samples in pooled testing results in increased Ct values for each viral target gene.46,47 For instance, pooling at a dilution of 1:10 led to a median loss of Ct values of 2.87 for the E gene, 3.36 for the RNA-dependent RNA polymerase gene, and 2.99 for the N gene.47 When conducting sample pooling, one must keep in mind that borderline positive samples may escape detection and return false-negative results. To adjust for this, a possible solution may be to add a few cycles to the threshold to minimize the chances of missing otherwise positive cases. The studies mentioned above assessed pooled sample testing using nasopharyngeal swab samples. Other studies have demonstrated the usefulness of pooled sample testing for evaluating saliva samples.48,49,50 Because the demand for screening large numbers of people is increasing, we have started comparing the sensitivities of pooled-sample testing and individual testing at Keio University. The results of the pooled-sample testing are beyond the scope of this paper, and the results we describe in the following section were obtained from individual RT-qPCR testing.
Keio University School of Medicine has been supporting Keio University Hospital by undertaking non-urgent COVID-19 screening testing, i.e., testing healthy and asymptomatic individuals who attended medical check-ups. This preserved the hospital’s capacity to test symptomatic patients and suspected individuals, to follow up patients already diagnosed, and to screen patients scheduled for treatment at the hospital. To achieve this, the Collaborative Research Resources, located within the medical school building, was registered by the Shinjuku City Public Health Center as an external clinical laboratory (ECL) to be an auxiliary COVID-19 PCR testing site in Tokyo in early May 2020. The ECL began to function in late August. At the end of December 2020, we had tested 2342 samples by RT-qPCR. Briefly, participants collected their own saliva samples at home in sterile cups and brought them within 1–2 days to the Center for Preventive Medicine at Keio University Hospital. After thermal inactivation of the virus and RNA extraction, RT-qPCR was performed according to the manufacturer’s instructions using the QuantStudio 5 Real-Time PCR system (Thermo Scientific, Waltham, MA, USA) and the SARS-CoV-2 Direct Detection RT-qPCR Kit (TaKaRa Bio, Shiga, Japan), which contains a primer/probe mix for the U.S. CDC 2019-nCoV_N1 and 2019-nCoV_N2 strains.51 We determined that samples with Ct values <40 with either primer were positive for SARS-CoV-2 infection. All procedures followed were in accordance with the ethical standards of the responsible committee at which this study was conducted (IRB approval number 20200063) and with the Helsinki Declaration of 1964 and later versions. Written informed consent was obtained from all patients and healthy individuals prior to data collection.
We had no positive cases in August, September, or October among the healthy and asymptomatic individuals who visited the Center for Preventive Medicine. During the third wave in Tokyo, we reported three asymptomatic cases in November. However, no positive cases were reported in December. The number of RT-qPCR tests performed on individuals visiting for medical check-ups at the Center for Preventive Medicine and the clinical characteristics of the three RT-qPCR-positive cases are shown in Tables 1 and 2.
| Month | PCR tests | Positive Cases |
|---|---|---|
| August | 162 | 0 |
| September | 526 | 0 |
| October | 617 | 0 |
| November | 542 | 3 |
| December | 495 | 0 |
aPCR screening started on August 24, which explains the smaller number of samples tested in August compared with that in other months.
| Case | Age (years) | Sex | Ct value of RT-qPCR | Prior exposure |
|---|---|---|---|---|
| Case 1 | 49 | Male | 29.0 | 7 days before |
| Case 2 | 54 | Male | 34.8 | Unknown |
| Case 3 | 45 | Female | 26.3 | Unknown |
Ct, cycle threshold.
Dental treatment can be high risk in terms of possible transmission of the virus because patients and healthcare providers are in close proximity and procedures can generate aerosols from patient saliva that may be mixed with blood. Aerosols can directly splash into the dentist/hygienist’s eyes or contaminate surfaces in the clinic.52 As a part of risk management efforts to provide a safe environment for dental care at Keio University Hospital, the ECL has been screening outpatients at the Department of Dentistry and Oral Surgery at Keio University Hospital who are scheduled to undergo treatment. From August to December, we tested 208 patients, and none had positive results.
The COVID-19 pandemic has affected countries worldwide and continues to have serious effects on public health and the economy. Rapid and accurate detection of infection is key to preventing transmission. Herein, we discussed the characteristics of the test modalities currently used to detect SARS-CoV-2. RT-qPCR is a highly sensitive and useful method for detecting ongoing infections, although it has limitations. Saliva samples are a good alternative to nasopharyngeal swabs, offering similar sensitivity and sample stability and saving human resources and PPE. Pooled-sample testing has proven effective in detecting SARS-CoV-2 infection in a large-scale setup, although we must keep in mind that borderline positive samples may escape detection in pools. We propose the new concept of “social cut-off” Ct values and risk stratification based on studies evaluating the correlation between Ct values and infectivity as a guide to safe levels of social activity with less stringent restrictions. Balanced ways of supporting public health and social and economic activities must be explored using a science-based approach.
H.N. conceived the study, and J.O. and H.T. wrote, revised, and edited the manuscript. M.S. and R.T. conducted RT-qPCR testing. S.M. and T.N. collected the samples from outpatients at the Department of Dentistry and Oral Surgery. H.T. collected samples from healthy and asymptomatic individuals who visited the Center for Preventive Medicine for medical check-ups. H.S. and K.M. provided resources for conducting RT-qPCR screening tests at the ECL facility. All authors have read and approved the manuscript.
The authors would like to thank Ms. Kaori Mochida, Ms. Emmy Yanagida, Mr. Hiroshi Yamada, Mr. Hiroumi Kurogi, and Ms. Matsumi Hirose for their technical assistance; Dr. Scott E. Woodman (Houston, TX, USA) for proofreading our manuscript; and Dr. A. Gordon Robertson (Courtenay, BC, Canada) for his helpful advice on data visualization and valuable manuscript discussions. The Keio Donner Project was launched in early 2020 to promote research to clarify the mechanism of COVID-19 disease, increase diagnostic test capacity, and develop effective therapeutic options. The name “Donner” is derived from the late Professor S. Kitasato, the founder of the Keio University School of Medicine. Special thanks to Masatoshi Wakui, MD, PhD; Yoshifumi Uwamino, MD, PhD; Toshinobu Kurafuji; Masayo Noguchi; Akemi Ohno; Hiromitsu Yokota, PhD; Haruhito Kikuchi; MD; PhD, Naoki Hasegawa, MD, PhD; Mitsuru Murata, MD, PhD; Yuko Kitagawa, MD, PhD; and Masayuki Amagai, MD, PhD for their contribution to the Keio Donner Project. This work was partially supported by the Keio University Global Research Institute (KGRI) Research Projects for New Coronavirus Crisis: Implementation of a Keio Model to Optimize SARS-CoV-2 PCR Tests through Systems Approach (PI: Koichi Matsuo); the Japan Agency for Medical Research and Development (AMED) (PI: Hiroshi Nishihara, Grant Number 20he1422004j0001); and the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) for utilization of the university’s PCR equipment. The funding agencies had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The authors have declared that no conflict of interest exists.