2017 年 58 巻 4 号 p. 596-599
2017 年 58 巻 4 号 p. 596-599
A large-scale spray pyrolysis apparatus in which the mist is pyrolyzed by a flame using a gas burner was developed for the mass production of Ag powder. Forty ultrasonic vibrators with a frequency of 1.6 MHz generated 4 dm3/h of mist. The thermal stability of the ultrasonic vibrators was improved using water-cooling. Spherical Ag powder was continuously produced, and the yield of the powders remained above 95% over 60 hours of continuous production with starting solution concentrations of up to 1.5 mol/dm3. Scanning electron microscopy (SEM) analysis showed that the particles were sub-micrometer in size with a dense microstructure, and they did not aggregate. Powder X-ray diffraction (XRD) demonstrated that the as-prepared Ag powder exhibited high crystallinity. The particle size of the Ag powder was approximately in agreement with the predicted size, calculated under the assumption that one Ag particle was formed from one droplet of mist. Moreover, 7 kg of Ag powder was produced over 12 h at 1.5 mol/dm3. This apparatus exhibited good reliability for the continuous mass production of Ag powder.
Recently, spherical Ag powders have attracted attention for use as electrode materials for electronic device applications such as multi-layer ceramic capacitors1,2), silicon solar cells3,4), and low-temperature co-fired ceramics5). For the development of these applications, the particles must be (1) spherical, (2) non-aggregated, and (3) chemically homogeneous. Fine Ag particles have already been prepared by liquid phase reactions such as chemical reduction6–8). However, the particles derived from these methods often agglomerate during drying. Moreover, liquid phase processes involve several prolonged steps, such as the chemical reaction, separation, and drying, and they lead to a high environmental cost because large amounts of solvent must be discarded.
Spray pyrolysis is a promising method for synthesizing Ag powders9–13) that satisfy the above particle requirements wherein a mist is pyrolyzed using an electric furnace. In particular, generating this mist using ultrasonic vibrators enables the formation of excellent sub-micrometer spherical particles with a narrow particle size distribution. However, each ultrasonic vibrator only generates a small amount of mist, but for the mass production of Ag powders, generating mist at a large scale is necessary. We therefore attempted to develop a large-scale powder production apparatus with forty ultrasonic vibrators. Using an electric furnace leads to high energy costs as the amount of mist increases, but the energy costs for mass production are expected to decrease by replacing the electric furnace with a gas furnace. However, Ag powders have not been mass produced by large-scale spray pyrolysis using a gas burner thus far. In this work, Ag powder was continuously produced by a large-scale gas combustion-type spray pyrolysis pilot apparatus, and the influence of the pyrolysis conditions on the Ag particle characteristics was investigated.
AgNO3 (Wako Pure Chemical Industries, Ltd.) was used as a starting material. First, AgNO3 was dissolved in distilled water at room temperature to prepare the starting solutions ranging in concentration from 0.1 to 2.0 mol/dm3. Figure 1 shows a schematic diagram of the gas combustion spray pyrolysis apparatus, which consisted of a mist generation unit (D × H × W = 300 mm × 400 mm × 300 mm) with forty ultrasonic vibrators, a gas furnace (450 mm × 4,500 mm) with twelve gas burners, and a powder collector with seven bag filters. The ultrasonic vibrators were water-cooled because the amount of heat they generate during long-term use weakens the vibration. The mist was generated from the starting solution by all forty ultrasonic vibrators at a frequency of 1.6 MHz and was then introduced to the gas furnace using air carrier gas at a flow rate of 0.16 dm3/s. The mist was continuously pyrolyzed to form Ag powder. Liquefied petroleum gas was used as the gas source. The flame temperature in the gas furnace ranged from 400℃ to 900℃ and was controlled by cooling using nitrogen gas. The flame temperature in the furnace was monitored by a thermometer with a Pt thermocouple. The formed Ag powder was collected by the bag filters. The efficiency of bag filter for collecting more than 100-nm-particles was 99%.
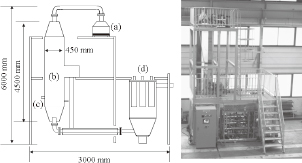
Schematic diagram and picture of the gas combustion type spray pyrolysis apparatus; (a) mist generation unit, (b) gas furnace, (c) gas burner, and (d) powder collector.
The average particle size, morphology, microstructure, and degree of aggregation of the as-prepared Ag powder were determined using an SEM (JSM-6390, JEOL). In the SEM pictures, 200 particles were randomly sampled to determine the average particle size and geometrical standard deviation (σg) of the as-prepared Ag powder. The crystal phase of the as-prepared Ag powder was observed by XRD (XRD-6100, Shimadzu) using Cu Kα radiation. Crystallite size of as-prepared Ag powder was determined from (111) plane by Scherrer equation. The specific surface area (SSA) of the as-prepared Ag powder was measured by the Brunauer–Emmett–Teller (BET) method using N2 adsorption (BELSORP-mini, BEL JAPAN).
Thus far, producing large amounts of Ag powder by spray pyrolysis has been difficult because ultrasonic vibrators generate only a small amount of mist. Therefore, a novel mist generation unit composed of forty ultrasonic vibrators was designed to generate a large amount of mist for the mass production of Ag powder. Figure 2 shows the effect of the number of the ultrasonic vibrators on the amount of mist generated, which increased with the increasing number of ultrasonic vibrators. Forty ultrasonic vibrators generated 4 dm3/h of mist. When ultrasonic vibrators more than forty are used, it is also possible to generate the mists more than 4 dm3/h.
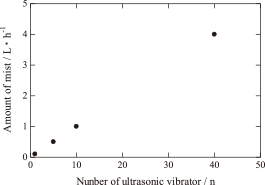
Relationship between the amount of mist and the number of ultrasonic vibrators.
The ultrasonic vibrators tend to heat up with increasing operation time, which often degrades the ultrasonic vibrator. In this work, the ultrasonic vibrators were cooled by water circulating in a copper pipe attached to the ultrasonic vibrators. Figure 3 shows the effect of the operation time of the ultrasonic vibrators on the temperature of the starting solution, both with and without cooling. When the ultrasonic vibrators were not cooled, the temperature of the starting solution increased up to 40℃ after 5 h, which is the rated temperature of the ultrasonic vibrator. On the other hand, cooling the ultrasonic vibrators maintained the temperature of the starting solution at 25℃ for 25 h.
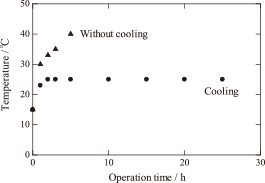
Relationship between the temperature of the aqueous solution and the operation time.
Ag powder was produced at temperature from 400℃ to 900℃. When AgNO3 was pyrolyzed at 400℃, the yield of as-prepared Ag powder was 85%. The yield increased to 95% when AgNO3 was pyrolyzed at or above 600℃. From the viewpoint of energy consumption, production at a lower temperature is desirable. Therefore, it was concluded that the optimum condition was 600℃.
Figure 4 shows SEM images and particle size distribution of the as-prepared Ag powder obtained at 600℃. These particles were non-aggregated and had a spherical morphology with a dense microstructure. No hollow particles were observed. The average particle sizes of as-prepared Ag powders obtained at starting solution concentrations of 0.3 mol/dm3 and 1.0 mol/dm3 were 0.4 μm and 0.7 μm, respectively. The geometrical standard deviations (σg) calculated from the two particle size distributions were both 1.3, indicating relatively narrow size distributions. This also suggests that the droplet size distribution of the mist is independent of the starting solution concentration and depends only on the frequency of the ultrasonic vibration.
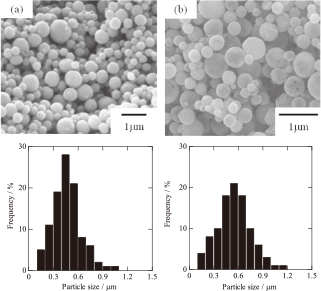
SEM images and particle size distribution of the as-prepared Ag powder obtained from starting solution concentrations of (a) 0.3 mol/dm3 and (b) 1.0 mol/dm3.
Figure 5 shows the relationship between the concentration and the particle size of the Ag powder. The black dots are experimental values, and the solid line was calculated based on an equation derived by Lang14) with a frequency of 1.6 MHz then 2.95 μm. The calculation assumed that one particle of powder was formed from one droplet of mist. Nagashima et al synthesized Cu particles by spray pyrolysis and introduced eq. (1)15) for determining the theoretical particle size of Cu, based on the above assumption.
| \[D_p = D_{drop} \left( \frac{CM}{1000 \rho } \right)^{1/3}\] | (1) |
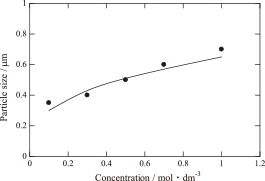
Relationship between the particle size of the as-prepared powder and the concentration of the starting solutions.
Therefore, we calculated the particle size of Ag powder by eq. (1) and examined the relationship between particle size and concentration. Both the particle size and calculated value increased with increasing concentration, as shown in Fig. 5. The measured particle sizes are almost in agreement with the predicted values. This result suggests that each mist droplet is uniformly pyrolyzed by the gas combustion. Also, it was conclude that one particle was generated from each droplet of mist without droplet coalescence or agglomeration. A thermogravimetry/differential thermal analysis (TG-DTA) confirmed that less than 1 mass% water was absorbed in all as-prepared powders. The nitrate and acetate completely decomposed, and then, NOX, CO2, and H2O gases were released. The H2O gas was considered to be adsorbed on the as-prepared particles in the bag filter. The SSA of the as-prepared Ag powder was about 1 m2/g, which suggested that the as-prepared particles had a dense microstructure and smooth surface.
The crystal phase and crystallinity of the as-prepared Ag powders were observed by XRD. In the XRD patterns in Fig. 6, the diffraction peaks of the as-prepared Ag powders were in agreement with those of Ag, and other phases were not observed. The crystallite size of the as-prepared Ag powder was 39 nm when the concentration was at 1.0 mol/dm3. This suggested that the Ag powder consisted of smaller polycrystals. The crystallinity of the as-prepared Ag powder increased with the increasing starting solution concentration. This results in many primary particles forming in the mist, which pyrolyze into metallic Ag and grow quickly due to sintering at higher concentrations.
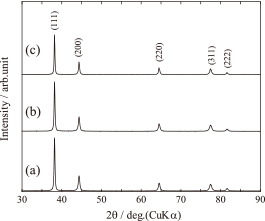
XRD patterns of as-prepared Ag powders obtained from starting solution concentrations of (a) 1.0 mol/dm3, (b) 0.5 mol/dm3, and (c) 0.3 mol/dm3.
To evaluate the production performance of the apparatus, the yield of Ag powder per hour was investigated. Figure 7 shows the relationship between the starting solution concentration and the production of Ag powder. The production of Ag powder increased linearly up to 615 g/h (95% of the theoretical yield) with the increasing concentration up to 1.5 mol/dm3. However, when the concentration was 2.0 mol/dm3, the production of Ag powder decreased to 510 g/h (60% of the theoretical yield) due to the decrease in the mist-generation rate because the starting solution had a higher viscosity at this concentration. When the concentration was 2.0 mol/dm3, the amount of mist decreased to 3 dm3/h. This resulted in a higher solution viscosity, which in turn led to the depression of ultrasonic vibration. From the viewpoint of production efficiency, the optimum concentration for producing Ag powder was 1.5 mol/dm3 with this apparatus, with a yield of over 95%. By operating this apparatus for 12 h per day with a concentration of 1.5 mol/dm3, it would be possible to produce approximately 200 kg of Ag powder per month.
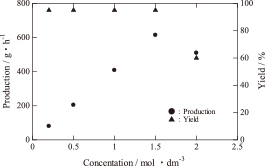
The production and yield as a function of the starting solution concentration after 12 h of operation.
In addition, the reproducibility of the apparatus was evaluated by measuring the particle size and yield during 60 h of continuous production. Figure 8 shows the changes in the particle size and yield as a function of production time. A sample was taken out from the bag filter every 12 h. When the concentration was 1.5 mol/dm3, more than 7 kg of Ag powder was produced every 12 h, and the yield of Ag powder remained higher than 95% over 60 h of production. The fluctuations in the particle size and yield were in the range of 5%, which suggested that the apparatus exhibited good stability for the large-scale and long-term production of Ag powder.
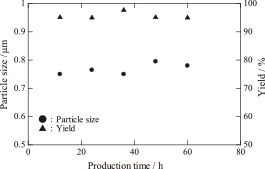
The particle size and yield as a function of the production time with a 1.5 mol/dm3 starting solution.
A large-scale spray pyrolysis apparatus with a gas burner was developed for the mass production of Ag powder. A mist of 4 dm3/h was generated by forty ultrasonic vibrators at a frequency of 1.6 MHz. The as-prepared Ag powder had a sub-micrometer size with a dense microstructure and high crystallinity. The various particle sizes were in good agreement with those predicted for each concentration. When the concentration was 1.5 mol/dm3 or less, the yield remained higher than 95%, thus enabling the production of 7 kg of Ag powder in 12 h at a starting solution concentration of 1.5 mol/dm3. The results from 60 h of continuous operation confirmed that this apparatus exhibited good reliability for large-scale and long-term production of Ag powder.