2019 年 60 巻 1 号 p. 61-67
2019 年 60 巻 1 号 p. 61-67
A large amount of sludge is generated from the neutralization of acid mine drainage (AMD) in Japan, which may cause severe environmental problems. This study showed there is potential to reuse such sludge in ceramic materials by thermal treatment. We sampled and characterized two types of iron-rich Japanese AMD sludge. Their phase compositions in the temperature range of 900–1200°C were identified by X-ray diffraction. α-Fe2O3 was the main crystalline phase in all sludge samples after thermal treatment. This can be used in ceramic enamels, porcelain bodies, and ferrite ceramics production. The results of leaching tests using acetic acid solution and distilled water as extraction fluids suggested that the stability of AMD sludge can be increased to a certain degree by thermal treatment; however, amorphous aluminosilicates formed by Al and Si impurities at lower temperatures weakened its stability. The enhanced mobility and volatilization of As impurity by thermal treatment also limits the application of AMD sludge. Applications that are insensitive to Al and Si impurities and products with higher arsenic stability are required when using sludges with high contents of impurities.
Acid mine drainage (AMD) is formed both in active and abandoned mines. The main source of AMD is oxidation of sulfide minerals when they are exposed to air and water during or after mining activities. Once AMD is formed, its generation can continue for hundreds of years, even when a mine is abandoned. Owing to its high acidity and high concentration of dissolved metals, AMD causes serious environmental problems for soils, water resources, and aquatic communities.1)
In Japan, AMD treatment plants are operated at approximately 80 of 5500 abandoned mine sites.2) Information concerning the production and composition of AMD sludge at these sites is currently limited. Zinck and Griffith surveyed AMD treatment and sludge management practices at 108 mine sites around the world, of which 66 sites were in Canada. The average annual production of dry sludge was about 9500 t, with production ranging from 20 to 135 000 t of dry sludge.3) By analogy, we estimated that large amounts of metal-rich sludges are produced during the treatment of AMD in Japan. To date, most AMD sludge in Japan has been deposited on land near mine sites. In addition to the problem of land occupation, the deposition of such sludge may result in severe pollution, such as risks of toxic metal migration from sludge to land, and the risk of sludge outflow caused by earthquakes. New technologies for AMD sludge recycling are therefore in high demand.
Previous studies on the reuse of AMD sludge include applications as: (1) an adsorbent to remove contaminants from wastewater and polluted soil owing to its high surface area; (2) a material to prevent and control AMD, because of its alkalinity; (3) alternative materials for use in cement, brick, and pigment production.4) For adsorbent applications, the possibility of producing more harmful materials and disposal of the final residue should be considered. For the second application, the effective component is residual alkaline neutralizer in the AMD sludge. This is only effective in the short term and risk of secondary pollution exists. For reuse as an alternative material, more valuable products are expected.
Metal-rich sludge has shown potential for application in value-added ceramic materials by high-temperature thermal treatment.5,6) The treatment also enhances metal stability of the sludge.7,8) In this study, we sampled and characterized two types of iron-rich AMD sludge from abandoned Japanese mine sites. Phase transformations of the sludge samples during thermal treatment were investigated by X-ray diffraction (XRD). Variations of leaching behavior after thermal treatment were evaluated using a dilute acetic acid (CH3COOH) solution and distilled water (DW) as extraction fluids. The potential and limitations of AMD sludge applied in ceramic materials are discussed.
Two types of iron-rich AMD sludge were sampled from abandoned Japanese mines. As shown in Fig. 1(a), Sludge A was generated from a CaCO3 and Ca(OH)2 two-step neutralization process. The excess sludge was dewatered by a plate filter press, followed by natural drying in an AMD treatment plant. We sampled naturally dried Sludge A from the plant. Sludge B was generated by aerated bacteria oxidation followed by CaCO3 neutralization (see Fig. 1(b)). Without dewatering and drying in the treatment plant, the excess sludge was naturally thickened in a sludge dam by gravity settling. We sampled Sludge B from the sludge dam.
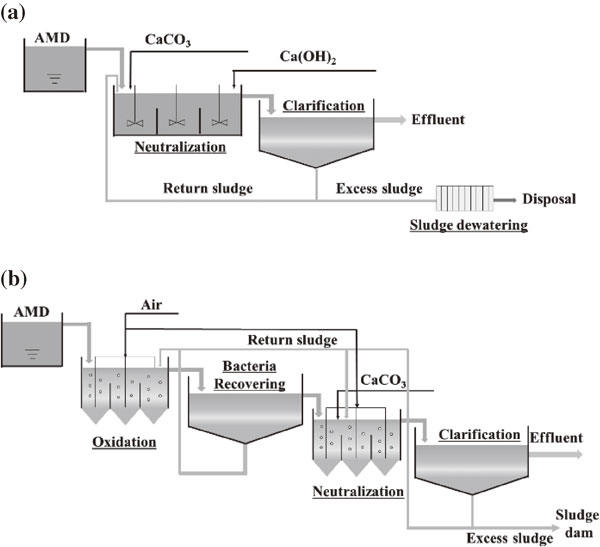
Simplified flowsheets for sludge generation from AMD neutralization treatments. (a) Sludge A and (b) Sludge B.
The moisture contents of the samples were determined using a gravimetric method. Their composition was analyzed after acid digestion using inductively coupled plasma atomic emission spectroscopy (ICP–AES) (Spectro Arcos, Ametek, USA). XRD patterns were recorded in the range 2θ = 10°–80° (0.02°/step, 0.5 s/step) using a Bruker D2 Phaser diffractometer with Cu-Kα radiation (30 kV, 10 mA) and a LYNXEYE detector (Bruker AXS GmbH, Germany). The morphologies were observed at 15 kV using a field-emission scanning electron microscope (FE–SEM, SU6600, Hitachi High-Technologies Corporation, Japan). The mass loss and evolved gas were analyzed by using a STA449F1 Jupiter simultaneous thermal analyzer (NETZSCH-Gerätebau GmbH, Germany) equipped with a JMS-Q1500GC mass spectrometer (JEOL Ltd., Japan).
The dried sludge samples were heated in an alumina crucible at 900, 1000, 1100, and 1200°C, and held for 2 h in a box furnace (KBF524N1, Koyo Thermo System Co., Ltd., Japan) in air. Following thermal treatment, the samples were cooled in the furnace and then ground to powder using a mortar and pestle.
XRD analysis of thermal treated sludge samples were performed under the same conditions as untreated samples. The leaching behavior of the samples before and after thermal treatment was evaluated using a dilute CH3COOH solution (toxicity characteristic leaching procedure (TCLP) extraction fluid 2#, pH = 2.88 ± 0.05)9) and DW as extraction fluids. The extraction vessels were agitated by a head-over-head rotator (RotoFlex Plus Tube Rotator, Argos Technologies, Inc., USA) at 30 rpm, 20°C, using a liquid/solid ratio of 20:1 and an extraction time of 18 h. The leachates were filtrated through a 0.45 µm syringe glass filter and adjusted to pH < 2 before analysis. The concentrations of the elements in the leachates were analyzed by ICP–AES. Their pH was determined using a pH meter (LAQUAtwin B-712, Horiba, Ltd., Japan).
The characteristics of AMD sludge depend on the nature of AMD and treatment process. The results showed significant differences between Sludges A and B.
Sludge A had a light brown color and 41.5% moisture content, whereas Sludge B was orange and contained 69.3% moisture. Table 1 presents the chemical compositions of the dried sludge samples. Both sludges were rich in iron. The sulfur content of Sludge B was more abundant than that of Sludge A. The contents of aluminum, silicon, calcium, and other impurities were obviously higher in Sludge A.

Figure 2 show the morphologies of Sludges A (a, b) and B (d, e), respectively. Both sludge flocs agglomerated to a few microns in length. Crystalline iron oxide or hydroxide was not identified in the mineral composition of Sludge A (Fig. 2(c)), which suggested that the majority of iron in Sludge A existed in an amorphous form. Excess neutralizer, in the form of Mg0.064Ca0.936CO3 (International Centre for Diffraction Data (ICDD), PDF #01-086-2335), remained. For Sludge B, Fig. 2(f) showed characteristic patterns of α-FeOOH (ICDD, PDF #01-075-5065).
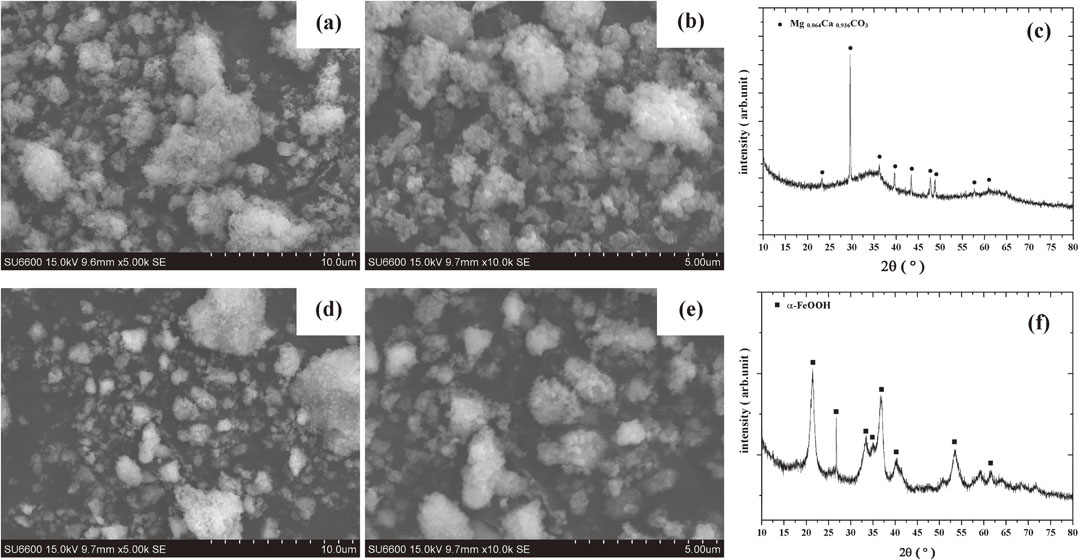
Micrographs and XRD patterns of dried sludge samples. (a), (b) micrographs, and (c) XRD patterns of Sludge A; (d), (e) micrographs, and (f) XRD patterns of Sludge B.
Results of thermogravimetric analysis with mass spectrometry (TG–MS) are summarized in Fig. 3. Sludge B showed larger mass loss (25.4%) than Sludge A (18.6%). The higher sulfate content of Sludge B is considered to be responsible for this phenomenon. The mass losses of evolved H2O (m/z = 18) were similar for the two sludges, but the differential thermogravimetry (DTG) and MS peaks differed significantly. Sludge A had one DTG (180°C) and one MS (183°C) peak. The loss of water was considered to correspond to dehydroxylation of amorphous iron hydroxide. Sludge B exhibited two DTG (122°C, 235°C) and MS (124°C, 240°C) peaks, which suggested that water was released in two steps during the heat treatment process. The second peak was believed to correspond to dehydroxylation of goethite, α-FeOOH. The first may have resulted from the removal of adsorbed water and loss of structured water from ferric sulfate.
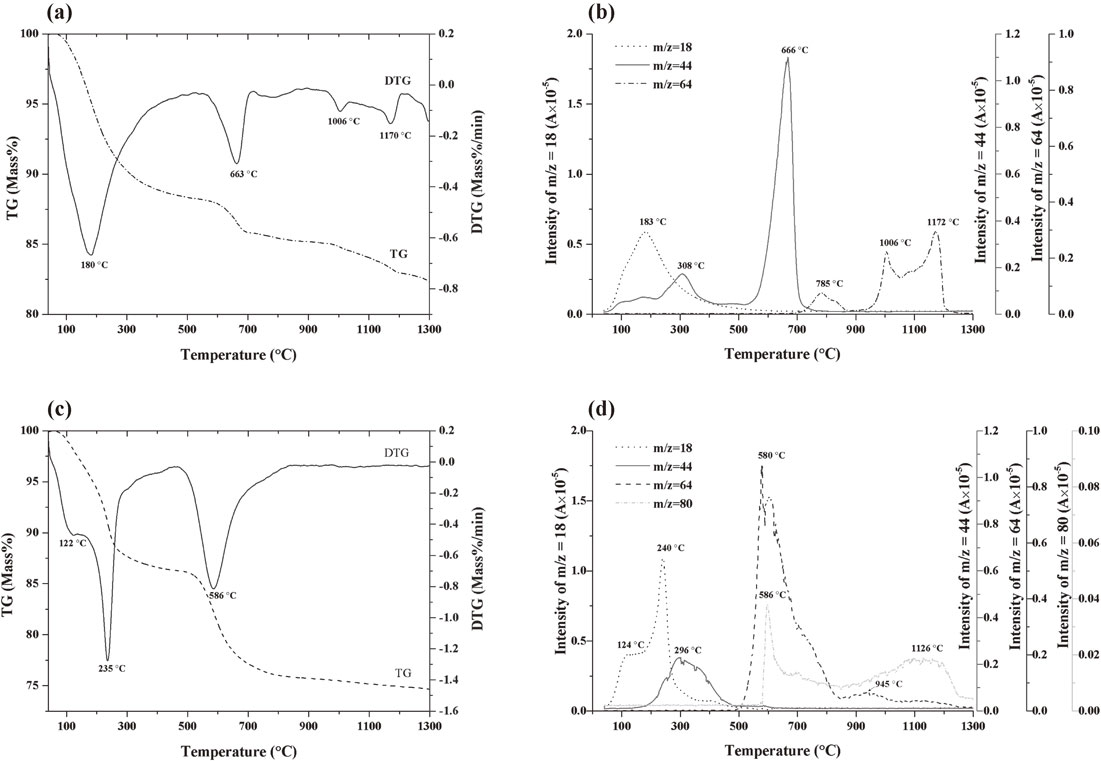
TG–MS results for dried sludge samples under simulated air atmosphere (20% O2 + 80% He). (a) TG–DTG and (b) MS curves of Sludge A, (c) TG–DTG and (d) MS curves of Sludge B.
The mass losses that occurred around 300°C (m/z = 44; CO2+) for both sludges are believed to correspond to decomposition of organic carbon compounds from flocculants used in the AMD treatment process. The losses around 600°C were contributed by carbonate (m/z = 44; CO2+) for Sludge A and iron sulfate (m/z = 64; SO2+ and m/z = 80; SO3+) for Sludge B. Losses above 1000°C are considered to correspond to the decomposition of calcium sulfate.
3.2 Effect of temperature on phase transformationThe hydroxides, carbonates, and sulfates in the AMD sludge samples decomposed during thermal treatment and new crystalline phases formed. Figures 4(a) and (b) show the XRD patterns of Sludges A and B after heating to 900, 1000, 1100, and 1200°C, respectively. α-Fe2O3 (ICDD, PDF #01-084-0308) was identified as the main crystalline phase in all samples. This formed by decomposition reactions of iron hydroxides or iron sulfate in the sludges.
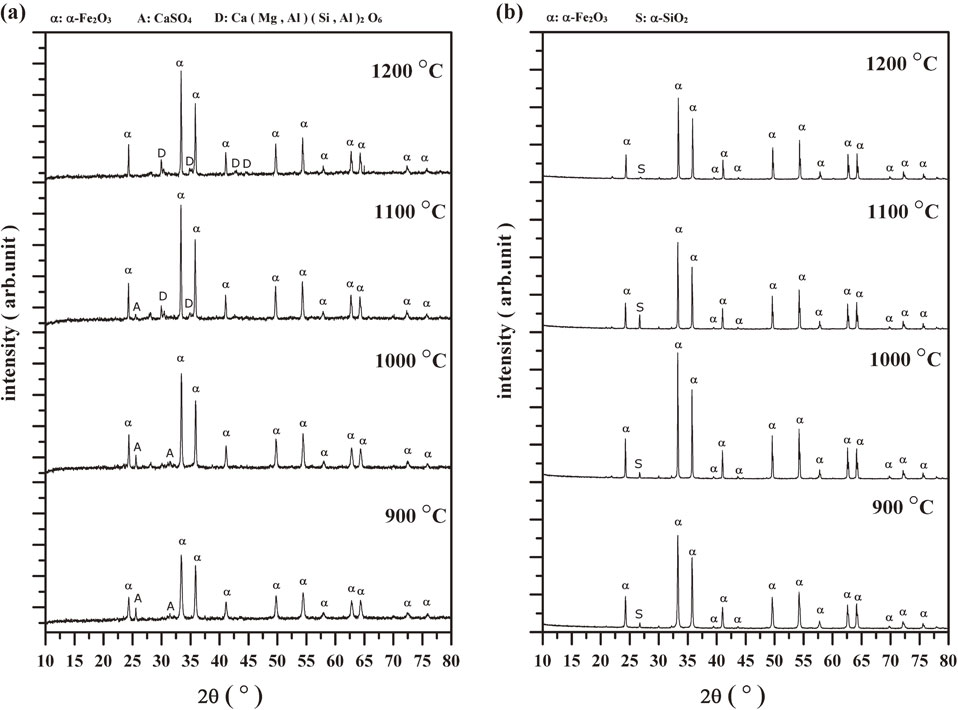
XRD patterns of sludge samples after thermal treatments at 900, 1000, 1100, and 1200°C. (a) Sludge A and (b) Sludge B.
For Sludge A, crystalline CaSO4 (ICDD, PDF #01-086-2270) was present at temperatures from 900 to 1100°C and disappeared when the temperature was increased to 1200°C. On the contrary, the aluminosilicate Ca(Mg,Al)(Si,Al)2O6 (ICDD, PDF #00-041-1370) started to be observed when the temperature was increased to 1100°C and the intensity of the corresponding peaks increased with increasing temperature. For Sludge B, α-SiO2 (ICDD, PDF #01-077-1060) existed as a second phase in the temperature range 900–1200°C. The maximum intensity of characteristic patterns of α-SiO2 was observed at 1100°C.
3.3 Effect of temperature on leaching behaviorThe sludge samples were leached before and after thermal treatment using CH3COOH solution and DW as extraction fluids. In all leaching tests, 0.5 g sludge sample and 10 ml extraction fluid were placed in extraction vessels. The concentrations of the main metal elements and arsenic in the leachates after leaching for 18 h are summarized in Fig. 5. Owing to the mass loss during thermal treatment (see Fig. 3), non-volatile elements in the treated samples were concentrated; therefore, their concentrations in the leachates should increase with increasing temperature if the leaching rates were same after thermal treatment. However, most curves in Fig. 5 did not show a monotonous rise. This result suggested that stability of AMD sludge can be increased to a certain degree by thermal treatment. Phase transformation during the treatment is believed to be responsible for it.

Concentrations of Fe, Al, Si, Ca, Mg, and As in leachates of sludge samples as a function of thermal treatment temperature.
For Sludge A, no significant change was observed with respect to the iron concentration in the leachate after thermal treatment. The highest aluminum and silicon concentrations in the leachates were observed in samples heated at 1000–1100°C. During thermal treatment, the hydroxides, carbonates, and sulfates in AMD sludge decomposed to related oxides. For sludge A with higher content of impurities, it is easy to form aluminosilicate species in thermal treated samples. The amorphous aluminosilicates formed at 1000–1100°C contributed to highest leaching rate of aluminum and silicon. Above 1100°C, crystallization of aluminosilicates as shown in Fig. 4(a) was responsible for the reduction of the leaching.
In the case of calcium and magnesium, formation of crystalline aluminosilicate was also considered to contribute to the enhanced stability. For Sludge B, the concentrations of almost all elements in the leachates decreased after thermal treatment. Significant drops in iron and aluminum concentrations from several hundreds to less than 10 mg/L were believed to be contributed by transforming iron sulfate, iron hydroxide, and aluminum hydroxide to their respective oxides.
In addition to the variations in phase stability, phase transformation also affected the pH of the leachate and further affected the leaching rate. As shown in Fig. 6, the pH values of the leachates changed considerately after thermal treatment. Residual alkaline neutralizer in Sludge A led to a rise in pH of the leachate. On the contrary, hydrolysis of iron sulfate in Sludge B may result in a drop. After thermal treatment, the pH values of the leachates of both sludge samples became similar, owing to the decompositions of carbonate and sulfate. The results also indicated that lower pH usually contributed to a higher leaching rate for the same phase, except for aluminosilicates. We presume that the pH difference between the untreated sludge samples was the main cause of the significant differences in iron and aluminum concentrations in the leachates, as shown in Figs. 5(a)–(d). Furthermore, Fig. 6(b) shows that the pH of the DW leachate of Sludge A increased after thermal treatment at 900–1000°C. This may be caused by decomposition of calcium carbonate and sulfate to form an intermediate calcium oxide. Dissolution of newly formed calcium oxide in DW led to a higher pH in the leachate, which further resulted in increased leaching rates of calcium, silicon, and aluminum. Associated with the transformation of calcium oxide to crystalline aluminosilicate above 1100°C, the pH and the calcium, silicon, and aluminum concentrations in the DW leachate decreased.

pH of leachates of sludge samples as a function of thermal treatment temperature using (a) CH3COOH and (b) distilled water as extraction fluids.
Different from the behavior of others, the leaching rate of arsenic was enhanced after thermal treatment of Sludge A. We presume that transformation of iron hydroxide to α-Fe2O3 led to this increased leaching rate. Especially when using dilute CH3COOH solution as the extraction fluid, the concentration of arsenic in the leachates increased significantly with increased heating temperature. During neutralization of AMD, arsenic can be coprecipitated with iron hydroxide. After thermal transformation of iron hydroxide to α-Fe2O3, the structure combined arsenic transferred to a surface-complexed one.10) The latter form is considered to be easier to release.
For Sludge B, the variation in the arsenic concentrations in the leachates was more complicated. As shown in Figs. 5(k) and (l), the arsenic concentration in the leachates rose after heating to 900°C, then fell when heated to higher temperatures. We considered that this is the result of volatilization of arsenic. Previous studies on the thermodynamics of arsenic show that CaO can effectively absorb As2O3 from the gas phase,11) whereas silicon and sulfur may enhance its volatilization.12,13) We believe that calcium in AMD sludge aids in reduction of the gas fraction of arsenic during thermal treatment, and silicon or sulfur impurities in the sludge may simultaneously weaken the effect of calcium. For Sludge A, with a higher calcium content, the volatilization of arsenic could be suppressed, whereas for Sludge B, with lower calcium and higher sulfur content, the effect of volatilization should be considered.
3.4 Potential and limitation of applications in ceramic materialsThe main crystalline phase of α-Fe2O3 present in AMD sludge samples after thermal treatment had a red color. This can be used as a pigment in ceramic enamels and porcelain bodies.14) Furthermore, there is great potential to use it as a raw material to fabricate value-added ferrite ceramics.15)
The common impurities in AMD sludge, such as Al and Si, may have negative effects on potential applications. For Sludge A, with a higher content of impurities, the influence of second phases, such as aluminosilicates, should be considered. Another limitation is the content of As in the AMD sludge. This could be easily volatilized during thermal treatment and residual As in treated sludge samples leached more easily. The composition of an AMD sludge is generally site-dependent. Sludge B, with a higher content of Fe and lower contents of Al, Si, and As is considered to have a wider range of applications in ceramic processing when compared with Sludge A.
Two iron-rich AMD sludges sampled from abandoned Japanese mines were heated at 900–1200°C to check the feasibility of their application in ceramic materials. We investigated the phase transformations and variations in leaching behavior after thermal treatment. The results indicated that it is possible to apply both sludges in ceramic enamels, porcelain bodies, and ferrite ceramics production; however, the content of impurities, especially the arsenic content, might limit their applications. The composition of AMD sludge varies between mine sites. For iron-rich AMD sludge, it is evident that sludge with higher iron content and less impurities (Sludge B) has greater potential for reuse. Applications insensitive to aluminum and silicon impurities and products with higher stability for arsenic will be studied further to improve the feasibility of using sludges with high impurity contents, such as Sludge A.
This study was supported by the Management Expenses Grants for National Universities Corporations “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and the Hatakeyama Culture Foundation. Part of this work was performed as joint research with the Metals Environmental Management Department, Japan Oil, Gas and Metals National Corporation (JOGMEC), and the authors acknowledge them for valuable discussions and support in sludge sampling. Mei Liu gratefully acknowledges support from the Japanese government (MEXT) for a doctoral scholarship. The authors thank the Fundamental Technology Center, Research Institute of Electrical Communication, Tohoku University, for the FE–SEM observations performed with a Hitachi SU6600 scanning electron microscope.