2019 年 60 巻 1 号 p. 111-120
2019 年 60 巻 1 号 p. 111-120
The thermodynamic properties for Nd2(MoO4)3 were investigated. Nd2(MoO4)3 is one of the end member of the yellow phases which are known as hygroscopic harmful phases in the nuclear fuel waste glasses. The standard molar entropy, 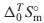 , at 298.15 K of Nd2(MoO4)3 was determined by measuring its isobaric heat capacities,
, at 298.15 K of Nd2(MoO4)3 was determined by measuring its isobaric heat capacities, 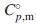 , from 2 K via the fitting functions including the Debye-Einstein formula and electronic- as well as magnetic terms. The Neel temperature, TN, estimated by extrapolating the magnetic-term in the fitting function. Its standard Gibbs energy of formation,
, from 2 K via the fitting functions including the Debye-Einstein formula and electronic- as well as magnetic terms. The Neel temperature, TN, estimated by extrapolating the magnetic-term in the fitting function. Its standard Gibbs energy of formation, 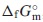 , was determined by combining
, was determined by combining 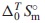 datum with the standard enthalpy of formation,
datum with the standard enthalpy of formation, 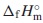 , which were estimated from ones for Ce2(MoO4)3 and Sm2(MoO4)3. The unknown standard Gibbs energy of solution,
, which were estimated from ones for Ce2(MoO4)3 and Sm2(MoO4)3. The unknown standard Gibbs energy of solution, 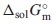 , at 298.15 K of Nd2(MoO4)3 was predicted from the reference data for MoO42−(aq) and Nd3+(aq). The obtained thermodynamic values are as follows:
, at 298.15 K of Nd2(MoO4)3 was predicted from the reference data for MoO42−(aq) and Nd3+(aq). The obtained thermodynamic values are as follows:
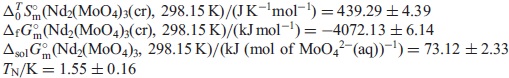
The data obtained in the present work are expected to be useful for geochemical simulations of the diffusion of radioactive elements through underground water.
This Paper was Originally Published in Japanese in J. Japan Inst. Met. Mater. 81 (2017) 485–493. To more precisely express the physical meaning of the functions for fitting the heat capacity, some their coefficients and thermodynamic values for Nd2(MoO4)3 were revised. The thermodynamic values for the relevant molybdates were revised based on the updated standard entropy at 298.15 K of molybdenum as the standard states. See Appendix describing the revised details.
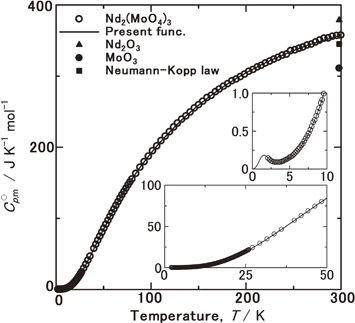
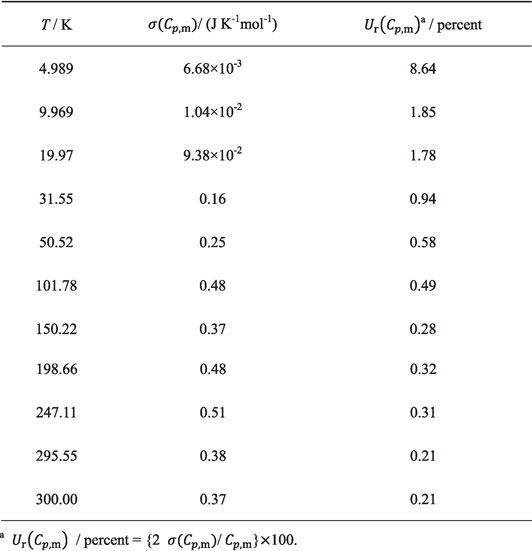
Fig. 2 Experimental molar heat capacities, C°p,m, for Nd2(MoO4)3 at 2–300 K (open circle) and ones calculated from the fitting functions (eqs. (4)–(7), solid line), compared with C°p,m(17/4 MoO3) (solid circle) and C°p,m(17/5 Nd2O3) (solid triangle) at 298.15 K. Solid rectangle shows C°p,m at 298.15 K on the basis of the Neumann-Kopp law.
Nuclear fuel wastes are immobilized in waste glasses via three steps: (1) they are dissolved in concentrated aqueous nitric acid; (2) uranium and plutonium are separated by a solvent extraction; (3) the remaining aqueous solution containing fission product elements, minor actinides (Np, Am, Cm) and structural materials is conditioned by calcining and the incorporation of dry material into borosilicate glass.1–7) Generally, a waste glass with such a high level of nuclear waste is then enclosed by an artificial barrier composed of steel, cement and bentonite and is disposed underground.1–7) However, direct contact between the nuclear waste glasses and ground water is assumed to be unavoidable due to the collapse of such an artificial barrier by unpredictable natural disasters such as the movements of the Earth’s crust, followed by diffusion of radio-active contaminated underground water. The Organization for Economic Co-operation and Development (OECD) Nuclear Energy Agency (NEA) Thermochemical Database (TDB) project provides a database of thermodynamic values of different compounds that contain the most significant elements that are relevant for nuclear waste safety management.8)
Molybdenum is generated as a fission product from uranium in the operating nuclear reactors. It is a harmful element because it forms hygroscopic crystals called the yellow phase in the borosilicate nuclear waste glass. The host crystal of the yellow phase is A2MoO4 (A: alkaline metals)3–5,7) and CaMoO4.5–7) There is immiscibility in alkali silicate9) and alkali borosilicate glasses.6) The MoO3 component in the borosilicate glasses expands the composition1) and temperature2) range of the immiscibility.1,2) Hyatt et al.3) clarified by X-ray absorption spectra that the MoO42− tetrahedra condensed for charge compensating against alkaline and alkaline earth metal cations, resulting in the precipitation of the yellow phases. Radioactive actinide and radio-activated rare-earth as well as transition metal elements are substituted into alkaline and alkaline earth metal sites in the yellow phases.7) Knowledge of the thermodynamic properties of the yellow phases is vital for understanding their phase stability.
In our previous studies,10–17) thermodynamic properties for the end members of the yellow phases MgMoO4,10) CaMoO4(cr),11) SrMoO4(cr),10,12) BaMoO4(cr),10,13) Sm2(MoO4)3(cr);14) Ag2MoO4(cr);15) their component MoO3(cr);11) and their aqueous species MoO42−(aq);10,11,16) the elemental molybdenum14) were investigated. Where mathematical expression of the Debye-Einstein formula to fit the experimental $C_{p,\text{m}}^{ \circ }$ data was investigated.10–17) The $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ data for the relevant molybdates FeMoO4,18–20) NiMoO4,21) ZrMo2O8,22–25) Th2MoO8,26,27) and UMoO628,29) were assessed10,11) by the present authors based on their experimental values.18–28) The measurements10–17) and assessments10,11) have been done as parts of the OECD NEA Mo TDB project.
In the present study, the standard molar entropy, $\Delta _{0}^{T}S_{\text{m}}^{ \circ }$, of Nd2(MoO4)3(cr) were determined by measuring the $C_{p,\text{m}}^{ \circ }$ data at temperatures of 2 K and higher using the relaxation method.10–17,21,30–42) The standard enthalpy of formation, $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$, of Nd2(MoO4)3(cr) was approximately estimated with the $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$ data for Ce2(MoO4)3(cr) and Sm2(MoO4)3(cr) which were determined by a solution calorimetry by Dash and Schkura.43) The standard Gibbs energy of formation, $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$, of Nd2(MoO4)3(cr) was approximately estimated by combing the determined $\Delta _{0}^{T}S_{\text{m}}^{ \circ }$ with the estimated datum of $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$. The phase stability of Nd2(MoO4)3(cr) in the nuclear fuel glass was investigated by comparing its $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ with them for MgMoO4,10) CaMoO4(cr),11) SrMoO4(cr),10,12) BaMoO4(cr),10,13) FeMoO4,10,11) NiMoO4,10) Ag2MoO4(cr),15) ZrMo2O8,10,11) Sm2(MoO4)3(cr),14) Th2MoO8,11) and UMoO6.11)
Felmy et al.44) measured solubility of Nd2(MoO4)3·XH2O(cr) into water. Kitamura et al.7) evaluated it standard Gibbs energy of solution, ΔslnG°, and solubility product, Ks. The unknown ΔslnG° datum of Nd2(MoO4)3(cr) was estimated from the $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ data for Nd3+ 45) and MoO42− 10,11) which was determined in our previous study. The estimated ΔslnG° of Nd2(MoO4)3(cr) was compared with one of Nd2(MoO4)3·XH2O(cr).
Nd2(MoO4)3(cr) was prepared from the following substitution reaction.
| \begin{align} &\text{2NdCl$_{3}{}\cdot$6H$_{2}$O(cr)} + \text{3Na$_{2}$MoO$_{4}$(aq)} \\ &\quad \rightarrow \text{Nd$_{2}$(MoO$_{4}$)$_{3}$(cr)} + \text{6NaCl(aq)} + \text{12H$_{2}$O(l)} \end{align} | (1) |
Table 1 shows details of the samples. Commercial powders of NdCl3·6H2O(cr) and Na2MoO4(cr) were used as the starting materials. The required amounts of the former and the latter were dissolved into water to form aqueous solutions, separately.
| \begin{equation} \text{2 NdCl$_{3}{}\cdot$6H$_{2}$O(cr)} \rightarrow \text{2Nd$^{3+}$(aq)} + \text{6Cl$_{3}{}^{-}$(aq)} + \text{12H$_{2}$O(l)} \end{equation} | (2) |
| \begin{equation} \text{3Na$_{2}$MoO$_{4}$(cr)} \rightarrow \text{6Na$^{+}$(aq)} + \text{3MoO$_{4}{}^{2-}$(aq)} \end{equation} | (3) |
The aqueous solutions were mixed to precipitate Nd2(MoO4)3(cr), expressed as eq. (1) by sum of eqs. (2) and (3). The suspension was filtered to recover the Nd2(MoO4)3(cr) precipitate which was heated to 413 K with heating rate 10 (K min−1) and dried at the temperature in air followed by furnace cooling. Because the dry Nd2(MoO4)3(cr) powder included NaCl(cr) as impurity. Such a NaCl(cr) was dissolved as NaCl(aq) by adding water again, followed by filtering to only recover the Nd2(MoO4)3(cr) precipitate. The Nd2(MoO4)3(cr) precipitate was dried at 413 K in air again. The dry Nd2(MoO4)3(cr) powder was then calcined at 973 K and for 2 h in O2(g) to stabilize its structure, followed by furnace cooling.
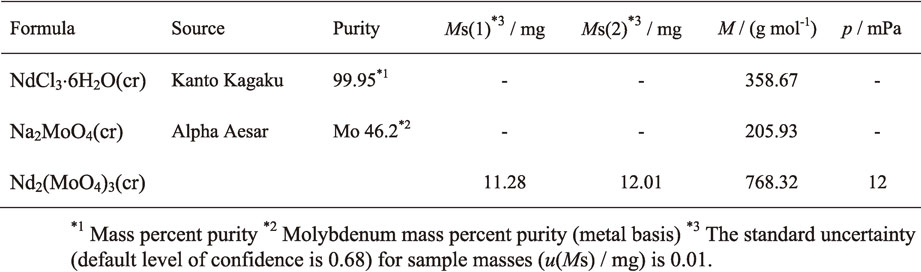
Figure 1 shows the XRD pattern of the synthesized Nd2(MoO4)3(cr) powder. A single phase of Nd2(MoO4)3 with monoclinic structure46) was confirmed. As described in section 3.3, since the solubility product, Ks, of the sample is negligibly small. This is reason for preparing the single phase of Nd2(MoO4)3 from the substitution reaction expressed as eq. (1).
XRD pattern of Nd2(MoO4)3 prepared in the present study.
The heat capacity, $C_{p,\text{m}}^{ \circ }$, of Nd2(MoO4)3 was measured from a very low temperature (2 K) to room temperature using a relaxation method instrument (model PPMS, Quantum Design, San Diego, CA)30,31) as in our previous studies.10–17,21,32–42) A sample is mounted with grease on a platform (3 × 3 m2) attached to a puck with eight fine Au–Pd wires, where a heater is used to supply a Joule’s heat pulse to the sample and a thermometer is attached to its bottom. The temperature increase provided by the heat pulse is measured by the thermometer. The $C_{p,\text{m}}^{ \circ }$ value of the sample can be determined as follows: (Step 1) the heat capacity of the platform with grease is measured using the heat pulse from the heater; (Step 2) the heat capacity of the sample and platform with grease is measured after mounting the sample on the platform. The $C_{p,\text{m}}^{ \circ }$ value of the sample can be calculated using PPMS software, which subtracts the results of the platform measurement (Step 1) from the sample and platform measurement (Step 2).
The Nd2(MoO4)3 powder was pressed in a steel die at 392 MPa to form a powder compact (10 mm × 2 mm). The powder compact was heated to 973 K with heating rate 10 (K min−1) and sintered 3 h and used for the $C_{p,\text{m}}^{ \circ }$ measurements.
Two samples for each substance were prepared by polishing the sintered bodies with emery paper to a thickness 0.70 mm. The details of the PPMS calorimetric measurements are summarized in Table 1. The sample masses (Ms/mg) for Nd2(MoO4)3 were 11.28 and 12.01. The standard uncertainty (default level of confidence is 0.68) of the sample masses (U(Ms)/mg) is 0.01. The molar mass (M/g mol−1) of Nd2(MoO4)3 is 768.32.
Each sintered sample was mounted on the platform by pushing softly with a fine Cu wire with ϕ0.2 mm to improve the thermal contact between each sintered sample and the platform. To verify such a thermal contact and microscopic homogeneity of the temperature increase due to the heat pulse that depends on the thermal conductivity in a sample body, the two Nd2(MoO4)3 samples were measured. Three series measurements for each sample were carried out, that is, six series measurements were performed for Nd2(MoO4)3. The average of the six series, i.e. the sum of each three series, was adopted as the result. The standard deviations, $\sigma (C_{p,\text{m}}^{ \circ })$, of the six measurements as the uncertainties based on the repeatability (default level of confidence is 0.68) and the ratios of $2\sigma (C_{p,\text{m}}^{ \circ })$ and $C_{p,\text{m}}^{ \circ }$, $U_{\text{r}}(C_{p,\text{m}}^{ \circ })$, as the expanded uncertainties with 0.95 of confidence for Nd2(MoO4)3(cr), were evaluated as measures of the thermal contact and microscopic homogeneity of the temperature increase.
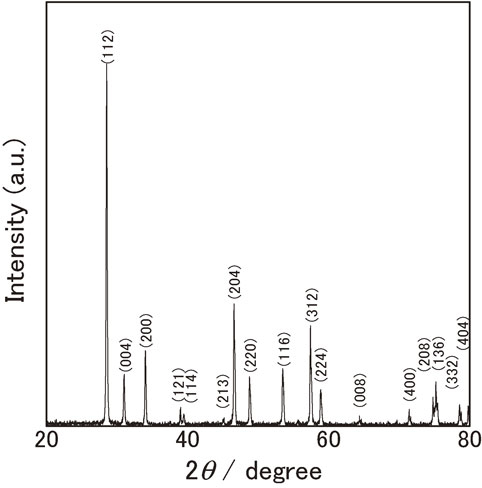
Figure 2 shows the measured $C_{p,\text{m}}^{ \circ }$ values (circle) for Nd2(MoO4)3 at 2–300 K. Over 20 K, it increased rapidly due to active lattice vibration. Below 20 K, they were found to be close to zero as a function of temperature, following the thermodynamic third law. However, below 4 K, $C_{p,\text{m}}^{ \circ }$ was found to increase with decrease of temperature. Such an increase of $C_{p,\text{m}}^{ \circ }$ appears to result from the Schottky anomaly of which a peak existed below 4 K. Mackintosh47) clarified that elemental neodymium is antiferro-magnetic from magnetic ordering of the magnetic moment on inter basal planes in d-hcp structure below 20 K by the neutron diffraction. Goff et al.48) clarified that such an inter basal plane antiferro-magnetic structure transform to inner basal plane antiferro-magnetism below 8 K by the neutron diffraction. In the crystal structure of Nd2(MoO4)3 with (I41/a), a highly anisotropic lamellar composed of the tetrahedral MoO44− are charge-compensated by modifying by Nd3+. In such a lamellar structure, the antiferro-magnetic phase transition appears to be caused from ordering magnetic moments of Nd3+ modifying the lamellar structure. The Néel temperature, TN, was estimated using a Schottky-like function as described later.
Experimental molar heat capacities, $C_{p,\text{m}}^{ \circ }$, for Nd2(MoO4)3 at 2–300 K (open circle) and ones calculated from the fitting functions (eqs. (4)–(7), solid line), compared with $C_{p,\text{m}}^{ \circ }$($\frac{17}{4}$ MoO3) (solid circle) and $C_{p,\text{m}}^{ \circ }$($\frac{17}{5}$ Nd2O3) (solid triangle) at 298.15 K. Solid rectangle shows $C_{p,\text{m}}^{ \circ }$ at 298.15 K on the basis of the Neumann-Kopp law.
The $C_{p,\text{m}}^{ \circ }$ data for MoO311) and Nd2O349) at 298.15 were compared with Nd2(MoO4)3. The number of moles of ions of Nd2(MoO4)3 is 17. Therefore, the $C_{p,\text{m}}^{ \circ }$ data for MoO3 (= 73.17 [J K−1mol−1])11) and Nd2O3 (= 111.332 [J K−1mol−1])49) were multiplied by (17/4) and (17/5), respectively. The average of $C_{p,\text{m}}^{ \circ }$(17/4 MoO3) and $C_{p,\text{m}}^{ \circ }$(17/5 Nd2O3) was calculated from Neumann-Kopp law. Figure 1 shows $C_{p,\text{m}}^{ \circ }$(17/4 MoO3) (solid circle), $C_{p,\text{m}}^{ \circ }$(17/5 Nd2O3) (solid try angle), and their average (solid rectangle). $C_{p,\text{m}}^{ \circ }$(Nd2(MoO4)3) was found to be higher than the value calculated from Neumann-Kopp law. The reason for such a deviation from Neumann Kopp law was described later.
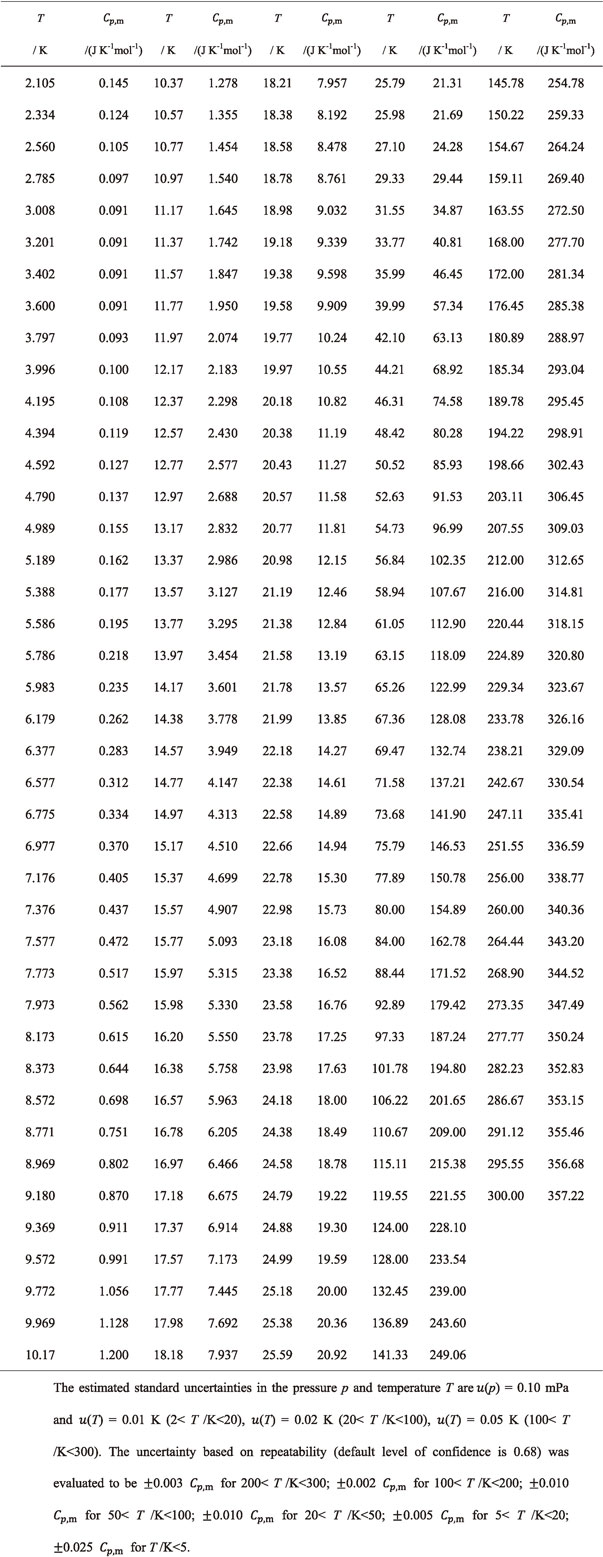
Table 2 shows the measured $C_{p,\text{m}}^{ \circ }$ data. Table 3 shows their standard deviations, $\sigma (C_{p,\text{m}}^{ \circ })$, of the six times measurements at representative temperatures as the uncertainties based on repeatability (default level of confidence is 0.68) and percent of twice $\sigma (C_{p,\text{m}}^{ \circ })$ against $C_{p,\text{m}}^{ \circ }$, $U_{\text{r}}(C_{p,\text{m}}^{ \circ })$, as the expanded uncertainties with 0.95 level of confidence as the error budget data.50) The $U_{\text{r}}(C_{p,\text{m}}^{ \circ })$ data were found to be 0.21 to 0.59% above 50 K, 1.85% at 9.97 K, and 8.64% at 4.997 K.
The measured $C_{p,\text{m}}^{ \circ }$ values were evaluated by the recently developed formulas:51–55) an empirical odd-power law function composed of the electronic and lattice vibration terms at 0 < T/K < 3.88; an empirical odd-power law function at 3.88 < T/K < 8.69; an empirical power law temperature function at 8.69 < T/K < 46.49; the Debye-Einstein function (DE) at 46.49 < T/K < 300.00 K, expressed as
| \begin{equation} C_{p,\text{m}}^{\circ} = \gamma_{1}T + \sum\nolimits_{i = 3,5,7,9}A_{i}T^{i} + B_{\text{fer}}T^{\frac{3}{2}}\exp\left(\frac{- \Delta}{T} \right) \end{equation} | (4) |
| \begin{equation} C_{p,\text{m}}^{\circ} = \gamma_{2}T + \sum\nolimits_{j = 3,5,7,9,11}B_{j}T^{j} \end{equation} | (5) |
| \begin{equation} C_{p,\text{m}}^{\circ} = \sum\nolimits_{k = 0}^{6}C_{k}T^{k} \end{equation} | (6) |
| \begin{equation} C_{p,\text{m}}^{\circ} = 3\mathrm{R}\left[m\mathrm{D}\left(\frac{\varTheta_{\text{D}}}{T} \right) + n\text{E$_{1}$}\left(\frac{\varTheta_{\text{E1}}}{T} \right) + l\text{E$_{2}$}\left(\frac{\varTheta_{\text{E2}}}{T} \right) \right] \end{equation} | (7) |
Table 4 shows the adjustable parameters. Six digits numbers for γ1, Ai, Bfer, Δ, γ2, Bj, Ck, ΘD, $\varTheta _{\text{E}_{1}}$, $\varTheta _{\text{E}_{2}}$, m, n and l are necessary to reproduce the experimental $C_{p,\text{m}}^{ \circ }$ data as five digits numbers on the basis of general error analysis.65) The $C_{p,\text{m}}^{ \circ }$ data calculated from the fitting functions with the coefficients given by Table 4 was shown as a solid line in Fig. 1. The present fitting functions reproduced the experimental values well. Below 2 K, by extrapolating the fitting function (eq. (4)), the configuration of the peak resulting from the Schottky anomaly caused by the antiferromagnetic phase transition was estimated. TN was estimated as eq. (8)
| \begin{equation} T_{\text{N}}/\text{K} = 1.55 \pm 0.16 \end{equation} | (8) |
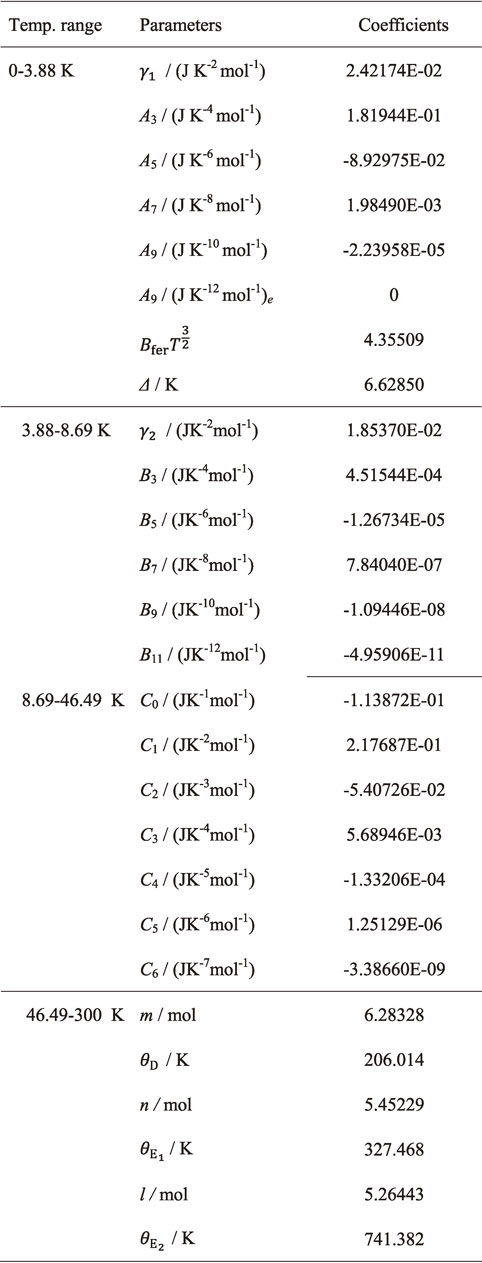
Figure 3 shows deviations, $U_{\text{fit}}(C_{p,\text{m}}^{ \circ })$, of the measured $C_{p,\text{m}}^{ \circ }$ data against the data calculated from the optimized Debye Einstein function (eqs. (4)–(7) and Table 4). The present fitting function was found to be consistent well with the experimental data within almost 0.5% above 25 K, within 1.2% at 10–25 K, within 6% below 10 K.
In our previous study,11) the deviation, $U_{\text{devi}}(C_{p,\text{m}}^{ \circ })$, between the data obtained by the adiabatic calorimetry and the relaxation calorimetry for Cu below 30 K and CaMoO4 at 50–300 K. $U_{\text{devi}}(C_{p,\text{m}}^{ \circ })$ was found to be 2.2% below 40 K; 0.86% at 50 K; 0.27% at 100 K; 0.09% at 150 K; 0.24% at 200 K; 0.32% at 250 K; 0.04% at 298.15 K.
From the fitting functions (eqs. (4)–(7) and Table 4), $C_{p,\text{m}}^{ \circ }$ at 298.15 K is concluded as
| \begin{align} &C_{p,\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(J$\,$K$^{-1}$mol$^{-1}$)}\\ &\quad = 356.52 \pm 1.31 \end{align} | (9) |
$\Delta _{0}^{T}S_{\text{m}}^{ \circ }$ was determined from the fitting function as
| \begin{align} &\Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/J$\,$K$^{-1}$mol$^{-1}$} \\ &\quad = 439.29 \pm 4.39 \end{align} | (10) |
Table 5 summarized the thermodynamic functions at 0–300 K as a function of temperature for Nd2(MoO4)3(cr): $C_{p,\text{m}}^{ \circ }$; $S_{\text{m}}^{ \circ }$; $H_{\text{m},T}^{ \circ } - H_{\text{m},298.15}^{ \circ }$; $S_{\text{m}}^{ \circ } - (H_{\text{m},T}^{ \circ } - H_{\text{m},298.15}^{ \circ })/T$.
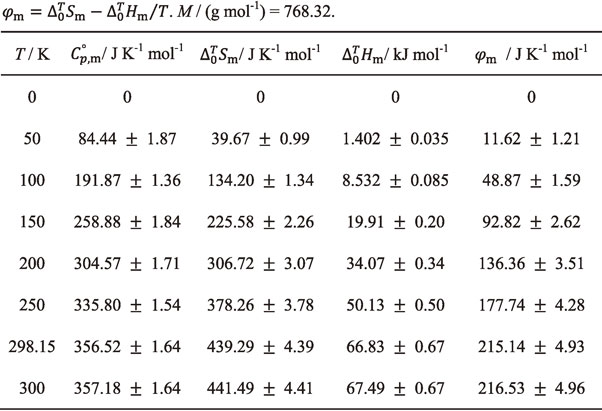
The standard entropy of formation, $\Delta _{\text{f}}S_{\text{m}}^{ \circ }$, of Nd2(MoO4)3(cr) at 298.15 K was determined to be
| \begin{align} &\Delta_{\text{f}}S_{\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(J$\,$K$^{-1}$mol$^{-1}$)} \\ &\quad = \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)}\\ &\qquad - 2 \times \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(Nd(cr), 298.15$\,$K)}\\ &\qquad - 3 \times \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(Mo(cr), 298.15$\,$K)}\\ &\qquad - 6 \times \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(O$_{2}$(g), 298.15$\,$K)}\\ &\quad = - 1019.51 \pm 9.45 \end{align} | (11) |
Dash and Shukla43) determined the $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$ data at 298.15 K for Ce2(MoO4)3(cr) and Sm2(MoO4)3(cr) by the solution calorimetry.
| \begin{align} &\Delta_{\text{f}}H_{\text{m}}^{\circ}\text{(Ce$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(kJ$\,$mol$^{-1}$)} \\ &\quad = - 4363.40 \pm 4.10 \end{align} | (12) |
| \begin{align} &\Delta_{\text{f}}H_{\text{m}}^{\circ}\text{(Sm$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(kJ$\,$mol$^{-1}$)}\\ &\quad = - 4388.70 \pm 3.60 \end{align} | (13) |
| \begin{align} &\Delta_{\text{f}}H_{\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(kJ$\,$mol$^{-1}$)} \\ &\quad = - 4376.10 \pm 5.46 \end{align} | (14) |
Combining $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$ with the $\Delta _{\text{f}}S_{\text{m}}^{\circ }$ obtained in the present study, the standard Gibbs free energies of formation, $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$, at 298.15 K were determined to be
| \begin{align} &\Delta_{\text{f}}G_{\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(kJ$\,$mol$^{-1}$)} \\ &\quad = - 4072.13 \pm 6.14 \end{align} | (15) |
The standard entropy of formation taking MoO3(cr) and Nd2O3(cr) as standard substance, $\Delta _{\text{f}}S_{\text{m,oxide}}^{ \circ }$, was calculated as eq. (16)
| \begin{align} &\Delta_{\text{f}}S_{\text{m,$\,$oxide}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)/(J$\,$K$^{-1}$mol$^{-1}$)} \\ &\quad = \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(Nd$_{2}$(MoO$_{4}$)$_{3}$(cr), 298.15$\,$K)}\\ &\qquad - \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(Nd$_{2}$O$_{3}$(cr), 298.15$\,$K)}\\ &\qquad - 3 \times \Delta_{0}^{T}S_{\text{m}}^{\circ}\text{(MoO$_{3}$(cr), 298.15$\,$K)}\\ &\quad = 54.43 \pm 5.09 \end{align} | (16) |
The phase stability of Nd2(MoO4)3(cr) in nuclear fuel waste glasses was examined, compared with the related alkaline earth molybdates MgMoO4, CaMoO4, SrMoO4, and BaMoO4, transition metal molybdates FeMoO4, NiMoO4 and ZrMo2O8, noble metal molybdate Ag2MoO4, rare earth molybdate Sm2(MoO4)3(cr), actinide molybdate UMoO6 and ThMo2O8.
In real nuclear fuel glass wastes, the reaction between the MmOn in glass component and the MoO3 in glass component in the glasses that precipitates the MmOn·l(MoO3)(cr) crystal should be discussed. Here l, m and n are 1, 1 and 1, respectively, for MMoO4 (M = Mg, Ca, Sr, Ba, Fe, Ni; MO·MoO3), and 2, 1, 1, respectively, for Ag2MoO4 (= Ag2O·MoO3), 3, 2, 3, respectively, for RE2(MoO4)3(cr) (RE = Nd, Sm: RE2O3·3MoO3), 2, 1, 2, respectively, for TMo2O8 (T = Zr, Th: TO2·2MoO3), 1, 1, 3, respectively, for UMoO6 (= UO3·MoO3).
| \begin{align} &\frac{1}{l}\text{M$_{m}$O$_{\text{${n}$ in glass}}$} + \text{MoO$_{\text{3 in glass}}$} \\ &\quad = \frac{1}{l}\text{M$_{m}$O$_{n}{}\cdot{}l$(MoO$_{3}$)(cr)}\quad \Delta_{\text{r}}G \end{align} | (17) |
ΔrG is defined from the standard Gibbs energy of the reaction, ΔrG°, and the activities, a, of the components in the glass.
| \begin{align} \Delta_{\text{r}}G & = \Delta_{\text{r}}G^{\circ} + \mathrm{R}T\ln \frac{(a_{\text{M${_{m}}$O${_{n}}{}{\cdot}{}{l}$(MoO${_{3}}$)(cr)}})^{\frac{1}{l}}}{a_{\text{M$_{m}$O$_{n}$ in glass}}{}^{\frac{1}{l}}a_{\text{MoO${_{3}}$ in glass}}}\\ & = \Delta_{\text{r}}G^{\circ} + \mathrm{R}T \ln Q \end{align} | (18) |
| \begin{equation} Q = \frac{1}{a_{\text{M$_{m}$O$_{n}$ in glass}}{}^{\frac{1}{l}}a_{\text{MoO${_{3}}$ in glass}}}. \end{equation} | (19) |
At equilibrium, ΔrG = 0, so that
| \begin{align} \Delta_{\text{r}}G^{\circ} & = - \mathrm{R}T \ln K\\ & = \frac{1}{l}\Delta_{\text{f}}G^{\circ}\text{(M$_{m}$O$_{n}{}\cdot{}l$(MoO$_{3}$)(cr))}\\ &\quad - \Delta_{\text{f}}G^{\circ}\text{(M$_{m}$O$_{n}$(cr))} - \frac{1}{l}\Delta_{\text{f}}G^{\circ}\text{(MoO$_{3}$(cr))} \end{align} | (20) |
Table 6 summarizes the $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$, ΔrG° and K data for precipitating Nd2(MoO4)3, compared with ones for MgMoO4,10) CaMoO4,11) SrMoO4,10) BaMoO4,10) FeMoO4,10) NiMoO4,11) ZrMo2O8,10) Sm2(MoO4)3,10) Ag2MoO4,10) ThMo2O811) and UMoO6.11) The phase stability of Nd2(MoO4)3(cr) was found to be more stable than that of Sm2(MoO4)3, Ag2MoO4, FeMoO4, MgMoO4, NiMoO4, UMoO6, ThMo2O8, and Zr2MoO8, while it was less stable than that of BaMoO4, SrMoO4 and CaMoO. Unfortunately, precipitation of Nd2(MoO4)3(cr) is not to be depressed by Zr2MoO8 which is the most advantageous with less solubility in water described in the section 3.3.

The standard Gibbs energies of solution, ΔsolG°, for $\frac{1}{l}\text{M}_{m}\text{O}_{n}\cdot l(\text{MoO}_{3})$ are defined by eq. (21).
| \begin{equation} \frac{1}{l}\text{M$_{m}$O$_{n}\cdot l$(MoO$_{3}$)} = \frac{m}{l}\text{M$^{+2(\frac{n}{m})}$(aq)} + \text{MoO$_{4}{}^{2-}$(aq)}\quad \Delta_{\text{sln}}G^{\circ} \end{equation} | (21) |
ΔsolG° is given by
| \begin{align} &\Delta_{\text{sln}}G^{\circ} \left(\frac{1}{l}\text{M$_{m}$O$_{n}{}\cdot{}l$(MoO$_{3}$)(cr), 298.15$\,$K}\right) \\ &\quad = \Delta_{\text{f}}G_{\text{m}}^{\circ}\text{(MoO$_{4}{}^{2-}$(aq), 298.15$\,$K)}\\ &\qquad + \frac{m}{l}\Delta_{\text{f}}G_{\text{m}}^{\circ}\text{(M$^{+2(\frac{n}{m})}$(aq), 298.15$\,$K)}\\ &\qquad - \frac{1}{l}\Delta_{\text{f}}G_{\text{m}}^{\circ}\text{(M$_{m}$O$_{n}{}\cdot{}l$(MoO$_{3}$)(cr), 298.15$\,$K)}\\ & \quad = - \mathrm{R}T\ln \frac{(a_{\text{M${^{+2(\frac{n}{m})}}$}})^{\frac{m}{l}}a_{\text{MoO${_{4}{}^{2-}}$}}}{(a_{\text{M${_{m}}$O${_{n}}{}{\cdot}{}{l}$(MoO${_{3}}$)}}^{\text{(cr)}})^{\frac{1}{l}}}\\ &\quad = - \mathrm{R}T\ln K_{\text{s}}, \end{align} | (22) |
Unknown ΔslnG° and Ks data were predicted by inserting into the $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ data for anion MoO42−(aq), cation $\text{M}^{ + 2(\frac{n}{m})}(\text{aq})$, and solid crystal MmOn·l(MoO3)(cr) into eq. (22). Where the $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ datum for anion MoO42− given by eq. (23)10) was used.
| \begin{equation} \Delta_{\text{f}}G_{\text{m}}^{\circ}\text{(MoO$_{4}{}^{2-}$(aq))/(kJ$\,$mol$^{-1}$)} = - 836.63 \pm 0.97 \end{equation} | (23) |
Table 7 shows the predicted ΔslnG° and Ks for Nd2(MoO4)3 and $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ for Nd3+, compared with ones predicted for MgMoO4,10) FeMoO4,10) NiMoO4,11) Sm2(MoO4)3,10) ThMo2O811) as well as UMoO6,11) and measured experimentally for CaMoO4,75) SrMoO4,75) BaMoO4,75) and Ag2MoO4.75) Nd2(MoO4)3 was found to be higher in ΔslnG° and lower in Ks than MgMoO4,10) FeMoO4,10) NiMoO4,11) Sm2(MoO4)3,10) ThMo2O811) as well as UMoO6,11) and CaMoO4,70) SrMoO4,70) BaMoO4,70) as well as Ag2MoO4,70) while it was found to be lower in ΔslnG° and higher in Ks than ZrMo2O8. That is, the most advantageous molybdate was found to be ZrMo2O8, implying that it is hardly dissolved into underground water. If ZrMo2O8 predominantly precipitates in the nuclear waste glass, the dissolution of MoO42−(aq) is suppressed. However, the phase stability of ZrMo2O8 was found to be lowest as described in the former section 3.2, meaning that it is hardly precipitates in the nuclear fuel waste glasses. Consequently, rapid cooling of the melts to produce the glasses is concluded to be necessary to depress formation of the molybdates including Nd2(MoO4)3.
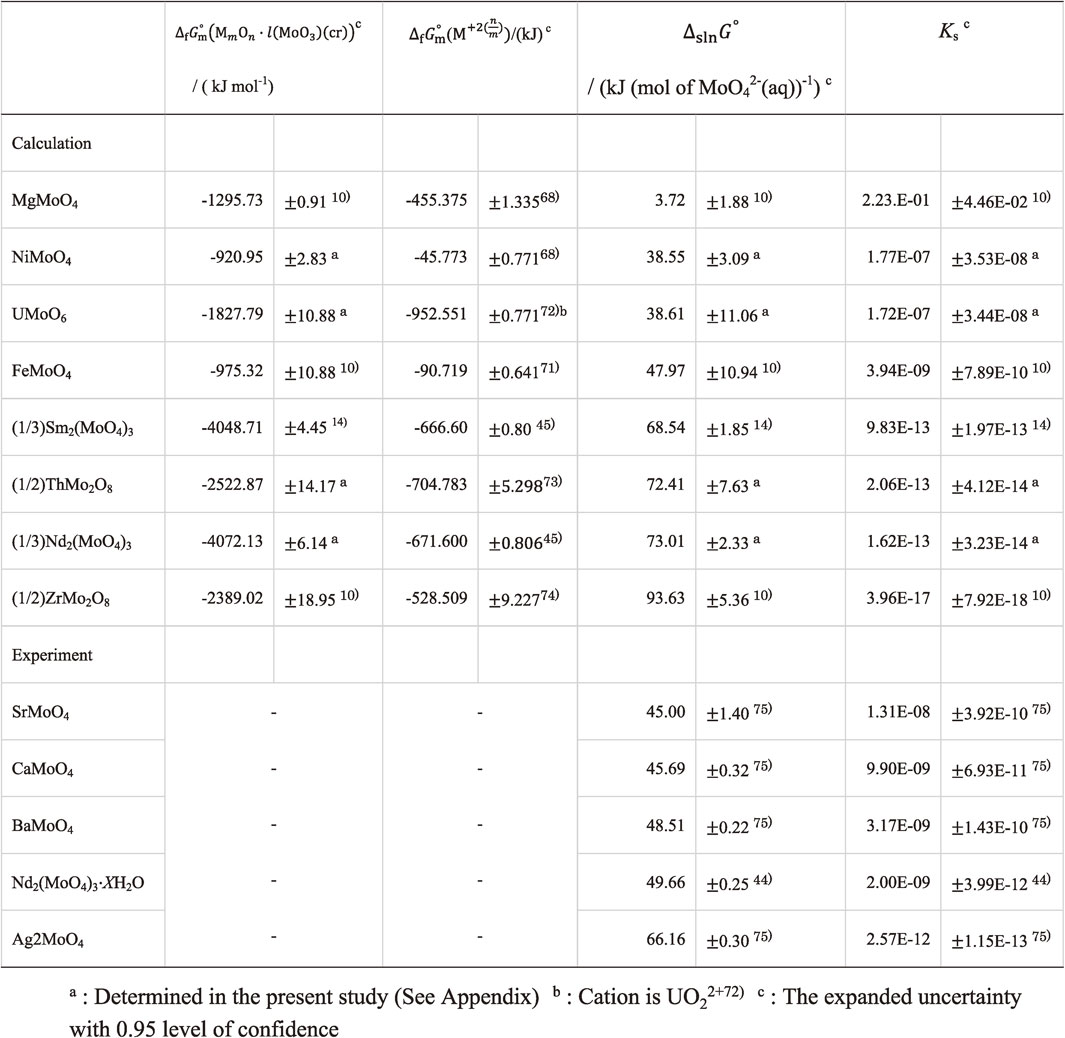
Felmy et al.44) measured solubility of Nd2(MoO4)3·XH2O44) into water. Kitamura et al.7) evaluated its ΔslnG° and Ks. The ΔslnG° and Ks data for Nd2(MoO4)3·XH2O44) was shown in Table 7. Nd2(MoO4)3·XH2O was found to be lower in ΔslnG° and higher in Ks than Nd2(MoO4)3, indicating that Nd2(MoO4)3·XH2O was equilibrated with water. Therefore, Nd2(MoO4)3·XH2O is likely to be formed when Nd2(MoO4)3 contacts with water, and it dissolves into water to form MoO42−(aq) and Nd3+(aq). The thermodynamic data for hydration from Nd2(MoO4)3 to Nd2(MoO4)3·XH2O should be further investigated.
The standard molar entropy, $\Delta _{0}^{T}S_{\text{m}}^{ \circ }$, at 298.15 K of Nd2(MoO4)3(cr) was determined by measuring its $C_{p,\text{m}}^{ \circ }$ at the temperatures of 2 K and above. The standard Gibbs free energy of formation, $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$, of Nd2(MoO4)3(cr) was determined by combining the $\Delta _{0}^{T}S_{\text{m}}^{ \circ }$ data with the literature datum of the standard enthalpy of formation, $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$, which was estimated from ones for Ce2(MoO4)3(cr) and Sm2(MoO4)3(cr). The unknown standard Gibbs energies of solution, $\Delta _{\text{sol}}G_{\text{m}}^{ \circ }$, at 298.15 K for Nd2(MoO4)3 were predicted from the literature data for MoO42−(aq) and Nd3+(aq). The Néel temperature, TN, estimated by extrapolating the magnetic-term in the fitting function. The obtained thermodynamic values are as follows: $\Delta _{0}^{T}S_{\text{m}}^{ \circ }$(Nd2(MoO4)3(cr), 298.15 K)/(J K−1mol−1) = 439.29 ± 4.39; $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$(Nd2(MoO4)3(cr), 298.15 K)/(kJ mol−1) = −4072.13 ± 6.14; $\Delta _{\text{sln}}G_{\text{m}}^{ \circ }$(Nd2(MoO4)3, 298.15 K)/(kJ (mol of MoO42−(aq))−1) = 73.12 ± 2.33; Ks(Nd2(MoO4)3, 298.15) = 1.62 × 10−13 ± 3.23 × 10−14; TN/K = 1.55 ± 0.16. The data obtained in the present work are expected to be useful for geochemical simulations of the diffusion of radioactive elements through underground water.
This article is published as English version of the article in Japanese published formerly as J. Jpn. Inst. Met. 81 (2017) 485–493. In Table 4 in the former article in Japanese, the coefficient of T3 term in the fitting function of the measured $C_{p,\text{m}}^{ \circ }$ data below 8.84 K was described as a negative value. However, at very low temperatures, the coefficient of T3 term is generally expressed as a function of the physicochemical constant Debye temperature.57,58) Therefore, the coefficient of T3 is positive is preferable. In the present English article, the two fitting functions at 0–3.88 K and 3.88–8.69 K in Table 4 were prepared so that the coefficients for T3 term were to be positive values.
The updated thermodynamic values for the elemental molybdenum14) as standard state was taken to re-evaluate the $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ datum at 298.15 K of ThMo2O8 in the present study. In our most recent study,10) the fitting functions for $C_{p,\text{m}}^{ \circ }$ SrMoO412) and BaMoO413) were revised as the most recent formula expressed in the present study, leading their updated $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$10) using the recent updated data for the elemental molybdenum.14) The $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ data for MoO3, CaMoO4, FeMoO4 and ZrMo2O8 assessed11) were updated using the up-dated data for the elemental molybdenum14) in our most recent study.10)
Morishita and Navrotsky21) determined $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ at 298.15 K of NiMoO4(cr) by combining $C_{p,\text{m}}^{ \circ }$ measured by the relaxation method with $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$ by the drop solution calorimetry. Where $C_{p,\text{m}}^{ \circ }$ was fitted by empirical power law functions. In the present article, $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ was up-dated by re-fitting $C_{p,\text{m}}^{ \circ }$ by the same functions expressed eqs. (4)–(7) and using the up-dated data for the elemental molybdenum14) as the standard state.
In our previous study,11) $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ at 298.15 K of UMoO6(cr) was assessed by combining its high temperature function by the transpiration method by Tripathi et al.29) with high temperature $C_{p,\text{m}}^{ \circ }$ by Dash et al.28) In the present article, $\Delta _{\text{f}}G_{\text{m}}^{ \circ }$ was updated by adding $\Delta _{\text{f}}H_{\text{m}}^{ \circ }$ and $\Delta _{0}^{T}S_{\text{m}}^{ \circ }$ at 298.15 K by Suleimanov et al.76) to them28,29) using the up-dated data for the elemental molybdenum14) as the standard state.
This research was funded in part by the Japan Society for the Promotion of Science (JSPS) under Grant-in-Aid for scientific research 26234567 and by Nuclear Energy Agency (NEA) Organization for Economic Co-operation and Development (OECD) under grant Phase IV of the thermodynamic data base project for nuclear waste management.