2019 年 60 巻 11 号 p. 2470-2474
2019 年 60 巻 11 号 p. 2470-2474
To propose an effective CuO-doped catalyst fabricated from red mud and rice hush ash for deep oxidation and emphasize the relation between properties and activity of catalysts, in this work, 5 mass% CuO-doped materials fabricated from red mud and rice husk ash were synthesized using urea/nitrate combustion technique. Effect of urea/nitrate molar ratios on properties and activity of prepared material in p-xylene deep oxidation was also investigated. The obtained catalysts were characterized by several techniques such as X-ray diffraction (XRD), Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET) surface area and Hydrogen Temperature Program Reduction (H2-TPR). The results showed that the change in urea/nitrate ratio could reduce CuO crystallite size of catalyst and enhance its reducibility leading to an increase of catalyst’s oxidative activity. The 5 mass% CuO-doped material with nanoparticles of about 20 nm synthesized with urea/nitrate molar ratio of 2 showed the best activity in p-xylene deep oxidation at the temperature range of 275–400°C. The deep oxidation of p-xylene to CO2 was almost complete at 400°C and WHSV of 12,000 mL·h−1·g−1. This study found an alternative, low-cost and friendly way for the exploitation of catalyst based on waste materials – red mud and rice husk ash. The modification of CuO-doped material by using urea as a fuel in the preparation is one of the most promising approaches, toward the development of effective VOCs oxidation. The cheap catalyst obtained in this work can be applied in the treatment of VOCs in polluted air.
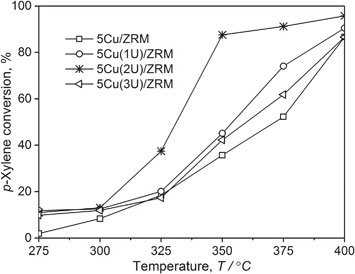
Fig. 5 The activity of catalysts in p-xylene deep oxidation.
Volatile organic compounds (VOCs) have been known as dangerous pollutants causing serious environmental and health issues. VOCs, known as greenhouse gases, may give rise in photochemical smog formation, ozone depletion, leading to global warming.1) VOCs are frequently released from various sources such as industrial processes and vehicle emissions.2) p-Xylene is one of VOCs which is primarily emitted from the petrochemical industry, oil refinery, automobile exhaust. Its vapor exposure over the recommended limit of 100 ppm may affect seriously on human health. There are many ways to remove VOCs including absorption, adsorption, thermal incineration, and deep oxidation.3) The deep oxidation is well recognized as one of the most appropriate methods for removing VOCs due to its high catalytic performance and moderate operating cost.3,4) Besides, the catalytic oxidation can treat VOCs efficiently at a very dilute concentration (below 1 vol%) with much lower operating temperature than thermal incineration.
Various catalytic materials such as precious metals2) and other transition metal oxides5–7) have been used for the deep oxidation of VOCs. Although precious metal catalysts (Pt and Pd) have high catalytic activity, they are quite expensive, limited and can be poisoned easily, and. Although catalytic activity of transition metal oxide catalysts (CuO, FeO, NiO, and MnO2) is lower than precious metal-based materials, they have high thermal resistance and not much sensitive. Therefore, the use of transition metal oxide in place of precious metal-based catalysts, especially CuO, is nowadays very attractive. Many materials such as SiO2, TiO2, NiOx, CoOx, Al2O3, Fe2O3, … have been researched for the synthesis of assisted CuO catalysts.8) The nature of these supports could significantly affect the reaction mechanism and catalytic performance. They have been demonstrated beneficial for VOCs deep oxidation. However, finding new supports with much low cost and rich porous structure has still been a challenge.
Red mud (RM) is a large-scale waste product, known as a hazardous material with high alkalinity from alumina production by Bayer’s process.9) Due to the relatively high presence of aluminum and iron, RM could be used as a potential alternative catalyst for various process.10) Up to now, RM has been used for many catalytic reactions, i.e. hydrogenation,11) hydro-dechlorination,12) catalytic combustion,9) hydrogen production,13) CO oxidation.14,15) The high surface area of acid-treated RM could enhance catalytic activity in CO oxidation.14,15) In other works, mesoporous CuO–Fe2O3 composite had exhibited high activity and thermal stability in CO removal,16) and CuO/Fe(OH)x catalyst had shown superior catalytic performance in preferential oxidation in the presence of H2.17) So, RM containing a large amount of iron can be used as a promising raw material for synthesizing CuO-doped catalytic material applied in deep oxidation of VOCs. Hu et al.14) had activated red mud by treating it in HCl followed by re-precipitation with NH3 and prepared CuO catalyst supported on activated RM (CuO/ARM) by deposition–precipitation for CO oxidation. 20 mass% CuO/ARM catalyst calcined at 200°C exhibited the highest activity and stability over 12 h time on stream. At tested conditions, CO conversion reached 100% at 170°C. However, in this work, a large amount of acid was used to neutralize NaOH in RM.
Rice husk ash (RHA), released from the rice husk combustion, is available in rice-producing countries as a waste. RHA is composed of about more than 90% of SiO2, and other trace elements like potassium, calcium, magnesium, iron, copper, manganese, zinc, etc.18) This composition of RHA and its low cost make RHA as a candidate for industrial applications.19) An attempt had been made to explore the synthesis of material with partial zeolite structure from RM and RHA at high pH. So, the alkalinity is unnecessary to be neutralized and the cost of this synthesis procedure will be cheaper than ever. In our previous work,20) catalytic nanomaterial with a part of zeolite structure (ZRM) having high activity in CO oxidation was synthesized from RM and RHA. The modification of ZRM with CuO increased active sites on the material surface, hence the catalytic activity was significantly improved. ZRM material containing 5 mass% CuO was found as the best catalyst for CO oxidation.
Recently, the usage of combustion method in preparation of catalysts has been attracted attention21) because of its advantages over conventional methods. These are low processing cost, simple and solvent-free method, quick preparation route. This combustion method leads to reducing particle size and high surface area materials.22) In general, organic compounds such as urea, citric acid, and glycine are employed as fuels and the metal nitrates acting as cation sources are used as oxidants.23) In the combustion process, the properties of obtained material including crystallite size, crystal phase, and surface area were dependent on the oxidant ratio/fuel.24)
In this study, CuO-doped catalytic material prepared from red mud and rice husk ash was synthesized by the urea-nitrate combustion method with the different molar ratios of urea/nitrate, where urea and nitrate salts were used as fuel and oxidants, respectively. The physicochemical characteristics of catalysts were investigated. The catalytic activity for p-xylene deep oxidation to CO2 and H2O was also evaluated.
The material (ZRM) was synthesized from RM red mud and RHA according to the process as detailed in the work.25) The main chemicals are Cu(NO3)2·3H2O (Xilong, >99 mass%), urea (Xilong, >99 mass%) and deionized water.
Catalysts CuO/ZRM were obtained by wet impregnation of the mixture solution of Cu(NO3)2 and urea on ZRM. The loading CuO was fixed at 5 mass%. The obtained suspension was dried and calcined as detailed in our previous work.20) The sample was stored overnight at room temperature before dried in air at 80, 100 and 120°C within 2 h at each temperature, then followed by the combustion process in the air at 600°C for 4 h. CuO-doped catalytic materials synthesized by urea–nitrate combustion method were denoted as 5Cu(xU)/ZRM, where x represents the molar ratios of urea/nitrate, x = 1, 2, and 3, respectively.
2.2 CharacterizationThe phase composition of the obtained catalysts was characterized by XRD method on apparatus Bruker D2 Phaser powder diffractometer with Cu Kα radiation (40 kV, 40 mA, and scanning step of 0.01°). Based on XRD result, the average crystal size of the sample at the highest peak was determined according to Scherrer’s equation:26)
| \begin{equation} d_{\textit{crys}} = \frac{K\lambda}{\beta\cos(\theta)} \end{equation} | (1) |
The specific surface area, pore volume and average pore size of catalysts were measured by BET nitrogen adsorption at −196°C (Quanta chrome Nova Win Machine). The morphology of synthesized samples was obtained using Emission Scanning Electron Microscopy (SEM, JEOL 7401), and Transmission Electron Microscopy (TEM, JEOL 1400). Characteristic of reduction was investigated by H2-TPR method using a Gas Chromatograph GOWMAC 69-350 with a thermal conductivity detector (TCD) as described in our previous work.20)
2.3 Catalytic activityBefore testing the activity, the synthesized catalysts were activated in airflow with the velocity of 200 mL·min−1 at 450°C for 4 h. The catalytic activity of samples in p-xylene deep oxidation was carried out in a micro-flow reactor under atmospheric pressure at the temperature range of 275–400°C. The concentrations of p-xylene and O2 in the stream were 0.34 and 10.5 mol%, respectively. The weight hourly space velocity (WHSV) was 12,000 mL·h−1·g−1, and the mass of catalyst was 1.0 g with the size range of 0.25–0.50 mm.
Gas mixtures in the input and output flow of the reactor were analyzed on a gas chromatograph of Agilent Technologies 6890 Plus using a FID detector, capillary column DB 624 (30 m length; 0.32 mm outer diameter; 0.25 µm thickness). The tests were undertaken in triplicate to ensure accuracy of the results.
XRD patterns of the catalysts (Fig. 1) showed the presence of several peaks at 2θ = 24.4, 29.8, 34.5, 36.1, 41.3, 49.8, 54.4, 63.5 and 64.5° with the highest intensity at 2θ = 34.5 and 36.1°.14) The typical reflections of hematite (Fe2O3) could be observed at 2θ = 24.4, 29.8, 34.5, 36.1, 41.3, 54.4, 63.5 and 64.5°;27) the peak at 2θ = 34.5° may also indicate the presence of rutile phase of TiO2, calcite (CaCO3). The reflection peak at 2θ = 24.4° also represents Quartz (SiO2). According to Huang et al.,28) the peaks of A-type zeolite could be observed at 2θ = 24.4, 29.8 and 36.1°. Besides, Volanti et al.29) proved that the typical reflections of CuO exhibited at 2θ = 36.1, 41.3, 49.9 and 54.4° in the XRD patterns. So, XRD patterns of the samples showed that there were Fe2O3, TiO2, CaCO3, and SiO2 in red mud and rice husk ash, and zeolite and CuO synthesized. It could be seen that the intensity of peaks of the samples synthesized via the urea-nitrate combustion method (5Cu(1U)/ZRM, 5Cu(2U)/ZRM, and 5Cu(3U)/ZRM) was higher than that of 5Cu/ZRM sample prepared without urea.20) From XRD results, at the highest peak with 2θ = 36.1°, the average size of crystals of oxide composite in the samples was determined according to Scherrer’s equation. The average crystallite sizes of oxide composite in 5Cu(1U)/ZRM, 5Cu(2U)/ZRM, and 5Cu(3U)/ZRM catalysts were 20.0, 17.3, and 18.5 nm, respectively (listed in Table 1). These sizes are smaller than that of 5Cu/ZRM catalyst prepared without urea (21.3 nm).20) Among three catalysts prepared by the urea-nitrate combustion method, the 5Cu(2U)/ZRM sample has the smallest average crystallite size. On the other hand, the appearance of diffraction peaks on XRD pattern of 5Cu(2U)/ZRM being more clearly is also observed, especially 2θ = 49.1° and 49.9° characterized for Fe2O3 and CuO respectively.

XRD patterns of catalysts.

SEM and TEM images of CuO-doped catalytic materials (Fig. 2 and 3) showed that all samples with uniform distribution of particles were observed. TEM images showed that, with the same CuO content of 5 mass%, the structure of samples synthesized via the urea-nitrate combustion method was more porous than the sample without urea.20) It was obvious that the application of the urea–nitrate combustion method to prepare CuO-doped catalytic materials synthesized from red mud and rice husk ash resulted in a better surface morphology and particles with similar size. These facts proved the positive effect of combustion method on the characteristics of the catalysts which could lead to better catalytic performance. Comparison of 5Cu(2U)/ZRM and 5Cu(3U)/ZRM samples illustrated that increase of urea/nitrate ratio from 2 to 3 made dispersion worse. It could be found from TEM images that the samples consisted of nanoparticles, but the particle size of 5Cu(2U)/ZRM and 5Cu(3U)/ZRM catalysts, around 20 nm, were smaller and more uniform than 5Cu/ZRM sample. These results were consistent with the SEM images. Therefore, using the urea–nitrate combustion method to synthesize CuO-doped catalytic materials had significantly reduced the size as well as increased the synchronism of catalyst nanoparticles.

SEM images of catalysts; a) 5Cu/ZRM; b) 5Cu(2U)/ZRM; and c) 5Cu(3U)/ZRM.

TEM images of catalysts; a) 5Cu/ZRM; b) 5Cu(2U)/ZRM; and c) 5Cu(3U)/ZRM.
The BET specific surface area, average pore diameter, pore volume of 5Cu/ZRM synthesized with and without urea are also shown in Table 1. The BET specific surface areas of 5Cu/ZRM and 5Cu(2U)/ZRM are 35.4 and 29.5 m2·g−1. Compared with 5Cu/ZRM, 5Cu(2U)/ZRM catalyst had a smaller BET surface area and porosity (seen in Table 1), mainly due to the larger amount of gases evolved when more urea was present for combustion. This result is similar to the report of authors.30,31) On the other hand, it is also possible for 5Cu(2U)/ZRM catalyst having smaller metal oxide dispersed on the catalyst surface, that resulted in the reduced surface area and porosity.
As followed from H2-TPR profiles of 5Cu(xU)/ZRM (Fig. 4) and 5Cu/ZRM sample,20) it could be seen that there were three reduction peaks in the H2-TPR of all samples. The first peak (α1, the temperature range of <300°C) with a maximum at 250°C was assigned to the reduction of the bulk-like CuO phases32) while the second peak (α2, the temperature range of 300–450°C) with low intensity was attributed to the reduction of Fe2O3 to Fe3O4, and the third broad one (α3, the temperature range of >450°C) with a maximum around 700°C could be ascribed to the reduction of Fe3O4 to FeO or Fe.10) From the data in Table 1, the total amount of hydrogen consumed for the reduction of catalysts prepared by urea/nitrate combustion method is greater than that of one without urea. Besides, the amounts of hydrogen consumed for the reduction of copper oxide and iron oxide (α1 and α2) of these catalysts was also much higher. Among the catalysts synthesized using the urea, 5Cu(2U)/ZRM catalyst has the highest amount of hydrogen for the reduction process (α1 and α2). Additionally, being different from 5Cu/ZRM sample,20) these three peaks of 5Cu(xU)/ZRM catalysts synthesized by the urea–nitrate combustion method shifted to the lower temperature range. Another peak with lower intensity at Tmax = 210°C could be observed in H2-TPR profiles of 5Cu(xU)/ZRM samples representing the reduction of the highly dispersed copper oxide species.32) Besides, the maximum temperature of a broad peak at the range of 450–850°C was reduced from 700°C down to 650°C. This proved that copper oxide and iron oxide phases in 5Cu(xU)/ZRM catalysts had reduced more easily. This H2-TPR result is not similar to that of Hu et al.14) possibly due to the difference of catalyst preparations and raw RM materials.

H2-TPR profiles of catalysts.
The analysis results of gas mixtures on the output of the reactor pointed out that there were only CO2 and water vapor products detected in these experiments. This proved that p-xylene was oxidized deeply over all samples. The catalytic activity of various 5Cu(xU)/ZRM catalysts (x = 1, 2, and 3) summarized in Fig. 5 showed that the catalysts have high activity in p-xylene deep oxidation. On all samples, the p-xylene conversion was enhanced remarkably when the reaction temperature was risen from 275 to 400°C and reached over 85% at 400°C. Catalysts (5Cu(1U)/ZRM, 5Cu(2U)/ZRM and 5Cu(3U)/ZRM) showed higher activity than 5Cu/ZRM sample did. This could be explained by the fact that the catalysts synthesized by urea–nitrate combustion method had better dispersion, higher reducibility with smaller particle size. Among these samples, 5Cu(2U)/ZRM catalyst was the most active one and p-xylene conversion reached almost 95% at 400°C. This fact proved that 5Cu(2U)/ZRM is one of the materials having high activity for deep oxidation of p-xylene.

The activity of catalysts in p-xylene deep oxidation.
Urea-nitrate combustion is an effective technique to prepare CuO-doped catalytic material synthesized from red mud and rice husk ash. The effect of the molar ratio of urea and nitrate on properties and catalytic activity of CuO-doped ZRM materials in the p-xylene deep oxidation was emphasized. The sample prepared with the urea/nitrate molar ratio of 2 showed the highest catalytic performance in p-xylene deep oxidation with p-xylene conversion of 95% at reaction temperature of 400°C. The use of urea–nitrate combustion method for synthesizing CuO-doped catalytic material is one of the most promising approaches in the design of efficient local structures for catalysts at the molecular level, toward the development of effective VOCs oxidation. The results of this work have provided a successful way to completely treat industrial waste resources such as red mud, rice husk ash and VOCs pollutants that will affect our environment in the future.
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number 562-2018-20-01.