2020 年 61 巻 11 号 p. 2079-2082
2020 年 61 巻 11 号 p. 2079-2082
Although the binary Al–Ir cubic quasicrystalline approximant has been expected to be a narrow-gap semiconductor, it has not yet been produced because the presence of Al site vacancies causes excess hole doping. We suggest that high-pressure synthesis (HPS) can effectively reduce these vacancies. In this work, we investigated how HPS affected the structural and thermoelectric properties of an Al–Ir quasicrystalline approximant, finding that the sample made by HPS had a larger Seebeck coefficient than a sample made by conventional spark plasma sintering (SPS). Further, applying high pressure increased the lattice constant and measured Al composition by increasing the number of Al atoms in the Ir12 icosahedral cluster. These results show that HPS suppressed vacancies in the cluster, which doubled the dimensionless figure of merit zT.
This Paper was Originally Published in Japanese in J. Thermoelec. Soc. Jpn. 16 (2020) 139–143.
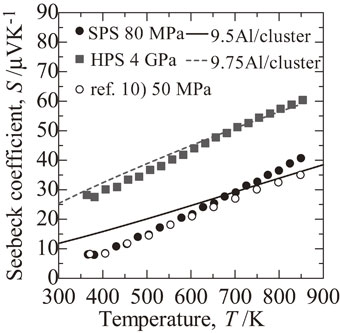
Temperature dependence of Seebeck coefficient S for Sample made by spark plasma sintering (SPS) (filled circle), high pressure synthesis (HPS) (square) and the sample of Ref. 10), respectively. Calculated S with 9.5 Al per cluster (solid line) and 9.75 Al per cluster (dashed line) are also shown.
The performance of thermoelectric materials is evaluated by the dimensionless figure of merit zT = S2σT(κel + κlat)−1, where S, σ, T, κel, and κlat are the Seebeck coefficient, electrical conductivity, absolute temperature, electronic thermal conductivity, and lattice thermal conductivity, respectively. For a material to have high zT, it must have a high power factor S2σ and a low κlat.
Aluminum–transition metal (Al–TM) quasicrystals and their approximants are potential thermoelectric materials because they show relatively high S2σ and low κlat (about 1 W m−1 K−1) due to the deep pseudo-gap in their density of states as well as their complex crystal structure.1–7) However, the highest zT reported in quasicrystals and approximants is 0.26 in Al–Ga–Pd–Mn quasicrystals,3) which is about a fourth of that of practical Bi2Te3 materials. Their zT is much lower because their S is at most 100 µV K−1, about half that of practical materials. To obtain a large S, a semiconducting quasicrystal with an electronic band gap of more than 6kBT (kB is the Boltzmann constant)8) is essential. However, no semiconducting quasicrystals have been found, and it is difficult to apply conventional first-principles calculations to quasicrystals because they lack periodicity.
In previous work, we searched for a semiconducting quasicrystalline approximant by applying first-principles calculations to a quasicrystalline approximant that has the same cluster as a quasicrystal and a periodic structure.7,9–11) The Al–Ir quasicrystalline approximant, which is the target material of the present study, has a structure that contains about 9–10 Al inside an icosahedral cluster of Ir, as shown in Fig. 1 (visualized by using VESTA 312)). In recent years, theoretical calculations performed by Mihalkovič et al. have shown that the Al–Ir approximant should be semiconducting with a band gap of about 40 meV if the cluster is filled with 10 Al.13) However, when such a sample was prepared and assessed experimentally, its thermoelectric properties showed metallic behavior rather than semiconducting behavior, so its thermoelectric performance was poor.10) Experimental and calculated results have suggested that the metallic behavior in this material originates from excess carriers (holes) caused by Al vacancies inside the cluster. Thus, suppressing these Al vacancies is key to producing a semiconducting Al–Ir quasicrystalline approximant. Although we prepared an Al-rich sample with a composition of Al73.5Ir23.5, Al3Ir—the adjacent phase on the Al-rich side—emerged. The difficulty of reducing Al vacancies by adjusting the composition was suggested by powder X-ray diffraction (XRD) measurements (Fig. 2).

Crystal structure of Al–Ir quasicrystalline approximant visualized using VESTA 3.

Powder XRD patterns of (a) Al73.3Ir26.7 and (b) Al73.5Ir26.5. Gray arrows stand for the peak of Al3Ir phase.
In the present study, we aimed to synthesize an Al–Ir quasicrystalline approximant with fewer Al vacancies inside the cluster by annealing it under high pressure, and we assessed its thermoelectric properties. In general, materials under high pressure tend to have a dense crystal structure. For example, the filled skutterudite compound AxB4X12 (A: alkaline earth metal element, rare earth element, etc.; B: group-VII or VIII transition metal element; X: pnictogen element) is a clathrate compound with a guest atom A inside the X12 icosahedral cluster, and it is often synthesized under high pressure.14–16) Thus, an Al–Ir quasicrystalline approximant with a cluster structure, such as filled skutterudite, might be filled with more Al in the Ir12 icosahedral cluster if it is synthesized under high pressure.
Ingots of the sample material were prepared using high-pressure synthesis (HPS; 700-ton Wakatsuki cubic press, Toshiba Corporation) or spark plasma sintering (SPS; SPS-515S, Sumitomo Metal Mining Co., Ltd.). To do so, raw powders of Al (4N, Kojundo Chemical Laboratory Co., Ltd.) and Ir (3N, Tanaka Kikinzoku Kogyo Co., Ltd.) were compacted into pellets with a composition of Al73.3Ir26.7 and melted in an arc-melting furnace. These ingots were homogenized by annealing at 1273 K for 72 h. We sintered them at a uniaxial pressure of 80 MPa for 20 min, because in our previous research10) on the same material, applying a uniaxial pressure of 50 MPa generated an insufficient relative density (95(2)%).
The HPS sample was powdered by the same method as SPS. The powder was densely packed into a cylindrical BN sample cell (inner diameter φ5 mm × height 6 mm) and capped with a BN disk (diameter φ5 mm × height 1 mm). The sample cell was inserted into a graphite heater (inner diameter φ6 mm × height 8 mm), and covered with a graphite disk (diameter φ7 mm × height 1 mm) on both sides. Then, a heater containing a sample cell was inserted into a cubic pyrophyllite pressure medium with a side length of 20.5 mm, and with a hole the same size as the outer diameter and height of the heater. Both ends of the heater were covered with molybdenum electrodes and pyrophyllite to produce a cubic pressure-medium cell. Here, the tolerances of all the HPS parts were kept within 50 µm, so that pressure could be applied homogeneously.
The pressure-medium cell was placed on the Wakatsuki cubic press, and hydraulic pressure of 4 GPa was applied for 1 h. Then, using a Joule heater, the sample was annealed at 1273 K for about 30 min. The temperature of the sample was confirmed using a temperature–resistance calibration table from the electric resistance, calculated from the current and voltage. After annealing, the heater was turned off, and the sample was left to cool for about 1 h. Then, the pressure was reduced for 1 h, and the cylindrical sample was removed from the sample cell inside the pressure-medium cell.
The phase compositions of the prepared samples were identified with an X-ray diffractometer (XRD; SmartLab, Rigaku Corporation, using CuK-L2, 3 lines) and a scanning electron microscope with an energy-dispersive X-ray spectrometer (SEM-EDX; JSM-6010LA, JEOL Corporation). The refinement of the lattice constant a and the composition of the sample were analyzed in the same way as in our previous studies.7,10) The true density of the sample was measured by helium displacement method using a dry pycnometer (AccuPyc1330, Micrometrics). The thermal conductivity κ of the sample was measured using the laser flash method (TC-7000, Advance Riko Co., Ltd.). The σ and S of the sample were measured by the DC four-probe method and the steady-state temperature difference method (ZEM-1, Advance Riko Co., Ltd.). Because the diameter of the HPS sample was small and it was difficult to measure κ, we assumed that the HPS and SPS samples had the same κlat, and we determined κ using the effective Lorentz number Leff from first-principles calculations (e.g., κ = LeffσT + κlat).
The first-principles calculations of S were done in the same way as in a previous study10) using a model containing 10 Al inside the cluster (10-phase), as proposed by Mihalkovič et al.13) The carrier concentration dependence of S was calculated using the rigid-band approximation, where the band structure does not depend on carrier concentration, and these results were compared with the measured S. Here, we assume that one Al atom supplies three electrons, and based the carrier concentration on the number of Al atoms inside the cluster, instead of on the number of electrons.
Figure 3 shows the powder XRD patterns of the annealed, SPS, and HPS samples, together with those from the previous study.10) No secondary phases appeared in any samples, indicating that a single phase remained after HPS. The half-width of the strongest peak in the XRD pattern of the HPS sample is about three times as broad as that of the annealed sample, suggesting that the crystallite may have been distorted by HPS.

Powder XRD patterns of (a) Al73.3Ir26.7 Ref. 10), (b) as annealed ingot, (c) SPS and (d) HPS sample.
Table 1 shows the compositions of the annealed, SPS, and HPS samples, obtained by SEM-EDX, as well as their lattice constants refined by Le Bail analysis. The HPS sample showed a larger lattice constant and a more Al-rich composition than the annealed and SPS samples, because the HPS caused the inclusion of more Al in the Ir12 icosahedral cluster. For the same reason, the HPS sample had a more Al-rich composition. It is not clear why the HPS sample was Al-rich even though all the samples had the same composition. Thus, we believe that Al3Ir—a compound on the Al-rich side from the quasicrystalline approximant phase—in the HPS sample changed to a quasicrystalline approximant phase, though it was in a small amount undetectable by XRD or SEM.

The relative density of each sample is close to 100% (SPS sample: 99.7 (6)%, HPS sample: 98.7 (3)%). To produce a high-density sintered sample of the Al–Ir quasicrystalline approximant, which has a high melting point of 1913 K,17) by SPS at 1273 K, we needed to sinter at a uniaxial pressure of about 80 MPa. Figure 4 shows the σ values for the SPS and HPS samples, revealing a metallic temperature dependence in both. The SPS sample showed 6100 S cm−1 at 350 K, which is 1.6 times the value (3700 S cm−1) of the Al–Ir quasicrystalline approximant prepared in our previous study.10) We attribute this difference to the greater uniaxial pressure during sintering (80 MPa) in the present SPS sample, producing a relative density of nearly 100%. However, the σ of the HPS sample is lower than that of the SPS sample, and the temperature dependence of the HPS sample is smaller than that of the SPS sample. These results suggest that the HPS sample not only has a lower carrier density than the SPS sample, but also that its electron relaxation time is shortened by a temperature-independent scattering mechanism such as impurity scattering. This scattering may come from lattice distortion, as suggested by XRD of the HPS sample.

Temperature dependence of electrical conductivity σ for SPS (filled circle), HPS (square) sample and the sample of Ref. 10) (open circle), respectively.
As shown in Fig. 5, the SPS and HPS samples both showed metallic behavior, with S increasing monotonically with temperature. The maximum S, at 850 K, was 40 µV K−1 for the SPS sample and 60 µV K−1 for the HPS sample, which was 1.5 times larger than that of the SPS sample. These results suggest that σ (S) decreased (increased) because of the decrease of the carrier (hole) density, caused by the suppression of Al vacancies by HPS. By calculating S using the 10-phase electronic structure, the S values in the SPS and HPS samples can be reproduced when the number of Al atoms in the first shell cluster is 9.5 and 9.75, respectively.
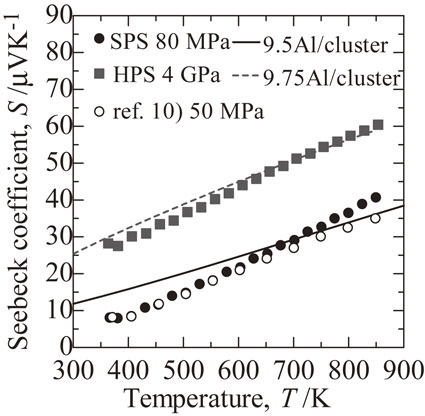
Temperature dependence of Seebeck coefficient S for SPS (filled circle), HPS (square) sample and the sample of Ref. 10), respectively. Calculated S with 9.5 Al per cluster (solid line) and 9.75 Al per cluster (dashed line) are also shown.
Figure 6 shows the temperature dependence of κ for each sample. The κ of the SPS sample was about 6 W m−1 K−1 across the entire temperature range. The calculated Leff, assuming the same carrier concentration as the calculated S, is lower than that of the Wiedemann-Franz law for both the SPS and HPS samples, as shown in Fig. 7. This behavior is similar to a previous result.7) The κlat of the SPS sample (calculated as κlat = κ − κel) is low, about 1 W m−1 K−1, across the entire temperature range, indicating that phonons are effectively scattered by the complex crystal structure of the quasicrystalline approximant. The κ of the HPS sample is about 3 W m−1 K−1, which is lower than that of the SPS sample, which occurred because the carrier (hole) concentration decreased and κel decreased.

Temperature dependence of total and lattice thermal conductivity κ and κlat for SPS (circle) and HPS (square), respectively.

Temperature dependence of effective Lorenz number Leff for SPS (filled circle) and HPS sample (square), respectively.
As shown in Fig. 8, the zT in both the SPS and HPS samples increased monotonically with increasing temperature, and the maximum zT values of the SPS and the HPS samples were 0.07 and 0.14, respectively, at 850 K. The Al vacancies were suppressed in the HPS sample, doubling its performance versus the conventional SPS sample. However, it is not yet a semiconductor because the suppression of Al vacancies was insufficient. To obtain a semiconductor in future work, it will be necessary to optimize the applied pressure, nominal composition, and annealing temperature in order to further suppress vacancies.

Temperature dependence of zT for SPS (circle) and HPS (square), respectively.
We used HPS to synthesize an Al–Ir quasicrystalline approximant with fewer vacancies, and clarified its thermoelectric properties. This material should be semiconducting, but has metallic properties due to Al vacancies inside the cluster. The HPS sample showed an Al-rich composition versus the sample made by conventional SPS (SPS: Al73.2(3)Ir26.8(3), HPS: Al73.8(3)Ir26.2(3)), indicating that the Al vacancies in the HPS sample were suppressed. The HPS sample showed a larger S than the SPS sample, and its zT was 0.14 at 850 K, double that of the SPS sample. However, it remains unclear whether the HPS was effective in suppressing atomic vacancies, but this technique might be effective for other thermoelectric materials that have a crystal structure with clusters.
This work was supported by a KAKENHI Grant-in-Aid (JP16H04489) from the Japan Society for the Promotion of Science. We thank Dr. Hiromasa Goto of the Institute for Solid State Physics, The University of Tokyo, and Dr. Kazuki Tobita of the Graduate School of Frontier Sciences, The University of Tokyo, for their advice on the using the HPS equipment used in this study.