2020 年 61 巻 4 号 p. 758-765
2020 年 61 巻 4 号 p. 758-765
Oxygen (O) contamination in titanium (Ti) is difficult to control using conventional Ti powder-metallurgy technologies, owing to the strong affinity between Ti and O. In this study, we developed a new sintering process that can remove O from Ti by placing Ti green and yttrium (Y) in molten salt. This study demonstrates that the O concentration in Ti can be reliably controlled in the range of 200–2000 ppm O by varying aY in the Y/Y2O3 equilibrium at 1300 K in NaCl–KCl (l), such that the sintering reaction of Ti powder simultaneously proceeds. Furthermore, it is also shown that the O concentration in Ti can be reduced to 30–60 ppm O in YCl3 (l) in the sintering process, when the Y/YOCl/YCl3 equilibrium is employed. This study demonstrates the feasibility of a new sintering process that can control the O concentration in Ti to approximately 30–2000 ppm O. The process ensures economical rationality because the cost of Y metal is negligibly small in recent years. By developing this process, inexpensive high-O-concentration Ti powder can be applied for fabricating the desired low-O-concentration Ti products.

Fig. 5 Schematic illustration of the new sintering process developed in this study showing control of oxygen concentration in Ti.
Titanium (Ti) and Ti-alloy (Ti–6 mass% Al–4 mass% V, etc.) have desirable properties such as low weight, high strength, corrosion resistance, and biocompatibility.1–4) Additionally, Ti has an abundant natural resource, and Ti material is expected to become a major industrial metal such as steel and aluminum.5,6) However, the current production costs of Ti products are much higher than those made from common metals so that the use of Ti is limited to high-value-added products for specific applications, such as aircraft parts.7) The high production cost of Ti products is caused by inefficient, specialized, and high-cost techniques to control the purity of Ti in smelting and machining processes. This is mainly because Ti has an extremely strong affinity for oxygen (O) at high temperatures.7)
Powder metallurgy of Ti (PM-Ti) is suitable for producing Ti products, because it can decrease the number of high-temperature processes in machining (a near-net-shaped process). In recent years, PM-Ti technologies (e.g., 3D-printing) fields have advanced rapidly worldwide. Moreover, many new smelting methods for Ti were developed to reduce smelting costs and designed to produce Ti powder.8–11) PM-Ti has attracted much attention as a critical technology to enable the widespread use of Ti in our society.
With conventional PM-Ti technologies, the increase of the O concentration in Ti during the sintering process is unavoidable owing to the strong affinity between Ti and O.12) As the O concentration in Ti increases, the tensile strength also increases because of the solid solution strengthening effect, but the ductility of Ti deteriorates significantly.13–17) During the manufacture of low-O-concentration Ti products, expensive pure Ti powder with low-O-concentrations (e.g., 200–400 USD·kg−1)18,19) is required as starting material, resulting in high production costs. Considering the current trade-offs between strength and ductility, it would certainly be beneficial to develop a new deoxidation technology to control the O concentration in Ti to 100–2000 mass ppm O. The deoxidation technology will establish a novel sintering process that can produce Ti products with the desired O concentration starting from an inexpensive high-O-concentration Ti powder.
Until recently, a variety of methods that can produce sintered Ti with low-O-concentrations have been proposed and developed (Table 1).20–34) Generally, the sintering process of Ti is conducted in an inert-gas atmosphere or a vacuum. However, as shown in Table 1, the O concentration in Ti is inevitably increased in inert-gas and vacuum states during the sintering process. This occurs, because the partial pressure of O2 (i.e., O2 potential), $p_{\text{O}_{2}}$, must be extremely low to directly remove O from Ti, owing to the strong affinity between Ti and O. Based on equilibrium theory, the relationship between the O concentration in β-Ti ([O]Ti (mass%)) and $p_{\text{O}_{2}}$ is expressed as follows:35)
| \begin{equation} \text{1/2 O$_{2}$ ($g$)} = \text{O (1$\,$mass%, in $\beta$-Ti)} \end{equation} | (1) |
| \begin{equation} \Delta G^{\circ}{}_{\text{1,Ti}} = - 2.303\,\mathrm{R}T \log (f_{\text{O}} \cdot \text{[O]$_{\text{Ti}}$}/p_{\text{O${_{2}}$}}{}^{1/2}) \end{equation} | (2) |
| \begin{equation} \Delta G^{\circ}{}_{\text{1,Ti}} = - 583000 + 88.5 T\ (\text{J})\ [1173\unicode{x2013}1373\,\text{K}] \end{equation} | (3) |
For example, when 1 mass% O (10000 ppm O) is dissolved in β-Ti at 1300 K, $p_{\text{O}_{2}}$ is determined to be as low as 2.5 × 10−38 atm (2.5 × 10−33 Pa). At such an extremely low O2 potential, direct removal of O from Ti during the sintering process is impossible in an inert gas or vacuum state. Therefore, Ti powder with low-O-concentration is greatly needed for conventional PM-Ti technologies. However, low-O-concentration Ti powder is expensive, because it is difficult to produce with high-purity using conventional technologies.
Based on this background, many researchers have focused on using deoxidizing agents in PM-Ti. In particular, calcium (Ca) metal is well known for its strong deoxidizing capability and its high vapor pressure. For example, in 1984, Majima et al. reported a sintering method for Ti powder in Ca vapor, but the O concentration in Ti increased during the process.21) Until recently, some researchers (e.g., Choi et al. and Oh et al.) had reported sintering methods for Ti in Ca vapor.24,28) Nevertheless, the sintering methods of Ti using Ca vapor are not widely used at an industrial scale, partly because it is challenging to efficiently remove the by-products of the deoxidation reaction (e.g., CaO (s)) from inside the sintered Ti body.
In molten salts, such as CaCl2 (l), which is stable at extremely low O2 potential, the solubility of the oxide ion (O2−) is high. Thermochemical and electrochemical deoxidation processes in molten salts are widely known as efficient techniques to remove O from Ti. Ca metal is widely known as an efficient deoxidizing agent for Ti in CaCl2, and many researchers have reported that the sintering reaction of Ti powders occurred in molten salt.28,36–43) Additionally, researchers have produced sintered Ti from TiO2 powder using an electrochemical process with molten salt. In 2007, Tripathy et al. produced sintered Ti with 1500 ppm O or less by applying a voltage between a TiO2 cathode and a carbon anode in CaCl2 (Fray–Farthing–Chen Cambridge method, FFC method).22,44) In the same year, Strezov et al.23) reported in a patent that they had produced sintered Ti with 500 ppm O or less using the FFC method. More recently, Hu et al. produced golf-club-sized sintered Ti using the FFC method.30,31)
To the best of our knowledge, there has been no report about producing sintered Ti with reliably lower than 400 ppm O, or of precisely controlling the O concentration in Ti in the range of 100–2000 ppm O. In conventional PM-Ti technologies, O concentrations in Ti increase during the sintering process and expensive Ti powder with low-O concentration is necessary to produce reliable Ti products. To solve this problem for PM-Ti, we focused on developing a new sintering process that controls the O concentration in Ti using the deoxidation techniques of pyrometallurgy.
In the past, the amounts of rare-earth (RE) metals production were small, and it was not economical to use RE metals for the deoxidation of Ti on an industrial scale. However, the demand for RE magnets, especially Nd–Fe–B magnets doped with Dy, has rapidly increased in recent years.45) Some RE metals, including Y, La, Ce, and Ho are produced as by-products of Nd and Dy. Industrially, the applications of these RE metals are limited and their demands are not expected to increase. Therefore, there will be a chronic oversupply of these RE metals in the future. Thus, the use of Y, La, Ce, and Ho metals for new deoxidation technology in PM-Ti ensures efficient utilization of natural resources and can be justified economically.
In PM-Ti fields, some researchers have reported that the mechanical properties of sintered Ti could be improved with small additions of RE compounds (RE oxides, RE hydrides, RE borides, etc.).46–48) Especially, the mechanical strengthening effect due to oxide particle distribution by internal oxidation of RE elements is actively studied.
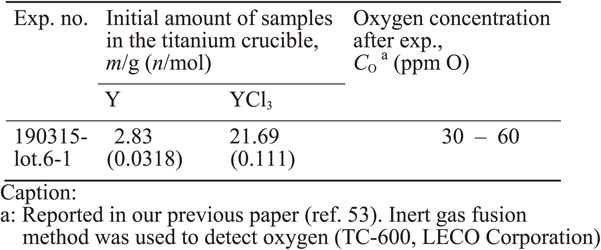
In contrast, we focused on the deoxidation of Ti by dissolving O in molten salt using RE metals. The deoxidation limits in the RE/RE2O3 equilibrium for bulk Ti have been experimentally demonstrated.49–53) Specifically, Y is considered to have the strongest deoxidizing capability in the RE/RE2O3 equilibrium in all rare earth metals. For example, when aY = 1 and $a_{\text{Y}_{2}\text{O}_{3}} = 1$ at 1300 K, the O concentration in bulk Ti decreases to approximately 100–200 ppm O in the Y/Y2O3 equilibrium. Just recently, we experimentally demonstrated that the O concentration in Ti could be decreased to 30–60 ppm O in the Y/YOCl/YCl3 equilibrium at 1300 K.53) However, there has been no research on the behavior of Ti powder at such an extremely low O2 potential ($p_{\text{O}_{2}} < 2.5 \times 10^{ - 42}$ atm at 1300 K, O concentration in Ti < 100 ppm O).
In this study, we developed a new sintering process that can remove O from Ti using Y metal as the deoxidizing agent in molten salt. Our goal is to demonstrate a new sintering process in which the O concentration in Ti can be controlled within the range of approximately 30–2000 ppm O (Table 1).
To design a new deoxidation method using molten salt, we determined the deoxidation reaction and the molten salt suitable to control the O concentration in Ti using a thermodynamic consideration. We set the experimental temperature to 1300 K, because this temperature is experimentally known to facilitate the deoxidation of Ti in molten salt.
In the Y–O–Cl system at 1300 K, Y2O3 (s), YOCl (s), and YCl3 (l) are reported as stable compounds. Figure 1 shows a phase stability diagram produced from a plot of the partial pressure of Cl2, $p_{\text{Cl}_{2}}$, vs. the partial pressure of O2, $p_{\text{O}_{2}}$, for the M–O–Cl system (M = Li, Na, K, Ca, Mg, Y) at 1300 K. Table 2 shows the thermodynamic data used to draw Fig. 1.35,54,55) The relationship between the O concentration in Ti and $p_{\text{O}_{2}}$ based on equilibrium theory, is also expressed in Fig. 1 using eqs. (1)–(3).


As shown in Fig. 1, LiCl, NaCl, KCl, and CaCl2 have regions coexisting with the Y/Y2O3 equilibrium (Line A) and the Y/YOCl/YCl3 equilibrium (Point B). Thus, in molten LiCl, NaCl, KCl, and CaCl2, Y metal is stable and the following deoxidation reactions proceed at 1300 K. In MgCl2, Y metal is unstable.
| \begin{equation} \text{O (in Ti)} + \text{2/3 Y ($s$)} = \text{1/3 Y$_{2}$O$_{3}$ ($s$)} \end{equation} | (4) |
| \begin{equation} \Delta G^{\circ}{}_{\text{r,$\,$deox [4]}} = 1/3 \Delta G^{\circ}{}_{\text{f,$\,$Y${_{2}}$O${_{3}}$}} - \Delta G^{\circ}{}_{\text{1,$\,$Ti}} \end{equation} | (5) |
| \begin{equation} \text{[O]$_{\text{Ti [4]}}$} = (a_{\text{Y${_{2}}$O${_{3}}$}}{}^{1/3}/a_{\text{Y}}{}^{2/3}) \exp(\Delta G^{\circ}{}_{\text{r,$\,$deox [4]}}/\mathrm{R}T) \end{equation} | (6) |
| \begin{equation} C_{\text{O,$\,$theo [4]}} = 200 \ (a_{\text{Y${_{2}}$O${_{3}}$}}{}^{1/3}/a_{\text{Y}}{}^{2/3})\ (\text{mass$\,$ppm O}) \end{equation} | (7) |
| \begin{equation} \text{O (in Ti)} + \text{2/3 Y ($s$)} + \text{1/3 YCl$_{3}$ ($l$)} = \text{YOCl ($s$)} \end{equation} | (8) |
| \begin{equation} \Delta G^{\circ}{}_{\text{r,$\,$deox [8]}} = \Delta G^{\circ}{}_{\text{f,$\,$YOCl}} - 1/3 \Delta G^{\circ}{}_{\text{f,$\,$YCl${_{3}}$}} - \Delta G^{\circ}{}_{\text{1,$\,$Ti}} \end{equation} | (9) |
| \begin{equation} \text{[O]$_{\text{Ti [8]}}$} = (a_{\text{YOCl}}/(a_{\text{Y}}{}^{2/3} \cdot a_{\text{YCl${_{3}}$}}{}^{1/3})) \exp(\Delta G^{\circ}{}_{\text{r,$\,$deox [8]}}/\mathrm{R}T) \end{equation} | (10) |
| \begin{equation} C_{\text{O,$\,$theo [8]}} = 3.4 \ (a_{\text{YOCl}}/(a_{\text{Y}}{}^{2/3} \cdot a_{\text{YCl${_{3}}$}}{}^{1/3}))\ (\text{mass$\,$ppm O}) \end{equation} | (11) |
Conversely, when Y metal is placed in molten MgCl2, the following displacement reaction occurs:
| \begin{equation} \text{3 MgCl$_{2}$ ($l$)} + \text{2 Y ($s$)} \rightarrow \text{2 YCl$_{3}$ ($l$)} + \text{3 Mg ($l$)} \end{equation} | (12) |
There is a possibility that the sintering Ti samples were contaminated by Y and other elements. For example, Y has a very small solubility in Ti (approximately 0.2 mol% at 1300 K) based on available thermodynamic data in the literature.56) This study is a proof-of-concept of the new sintering process with deoxidation, and the contamination of such elements should be investigated in detail as future works.
Figure 2 shows the relationship between [O]Ti and aY in the Y/Y2O3 and Y/YOCl/YCl3 equilibria at 1300 K. In this study, the Y/Y2O3 equilibrium was employed to control the O concentration in the range of 200–2000 ppm O with varying aY in eutectic NaCl–KCl (l) (50 mol% NaCl–KCl). Additionally, the Y/YOCl/YCl3 equilibrium was employed to reduce the O concentration in Ti to 30–60 ppm O in YCl3(l). The difference between the calculated value (3.4 ppm O) in eq. (11) of the O concentration in Ti and the experimental value (30–60 ppm O) was caused by the inaccuracy of ΔG°f, YOCl and $\Delta G^{\circ} {}_{\text{f},\,\text{YCl}_{3}}$ in the literature.53)

To vary aY in the Y/Y2O3 equilibrium and to control $p_{\text{O}_{2}}$ in the system, Y–Ag alloy was used in this study. Figure 3 shows the binary diagram of the Y–Ag system, and Fig. 4 shows the relationship between the mole fraction of Y, XY, in the Y–Ag alloy and aY at 1346 K.57) In this study, the relationship between XY and aY at 1346 K was applied as an approximation at 1300 K.
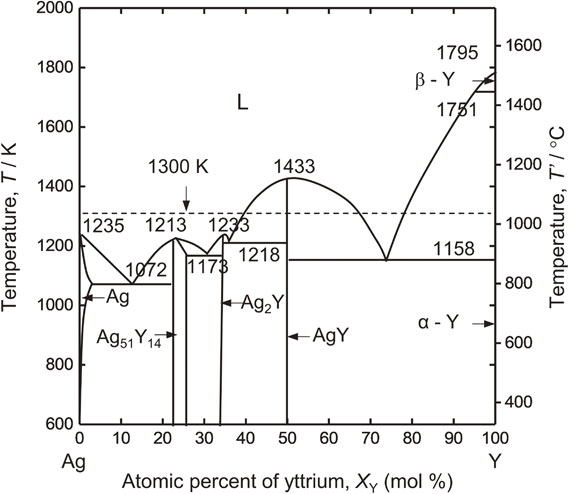
Y–Ag binary phase diagram.56)

Relationship between mole fraction of yttrium and activity of yttrium in Y–Ag system at 1346 K.56)
Through the thermodynamic considerations above, a new sintering process with the deoxidation of Ti using Y metal as the deoxidizing agent was designed, as shown in Fig. 5. In this process, with electron transfer in the conductive plate, O in Ti and Y dissolve into NaCl–KCl as oxide ion (O2−) and yttrium ion (Y3+), respectively. These ions produce Y2O3 (s) or YOCl (s) and the O concentration in Ti decreased following the reactions (4) and (8).
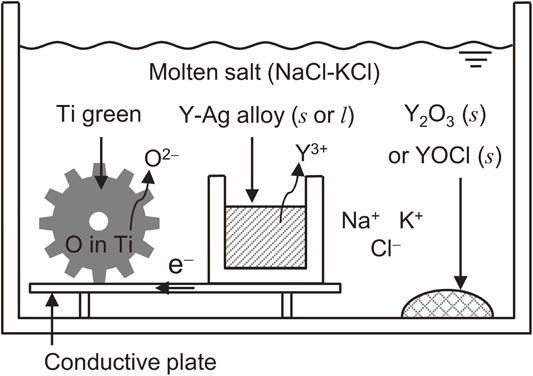
Schematic illustration of the new sintering process developed in this study showing control of oxygen concentration in Ti.
Figure 6(a) shows a schematic of the experimental apparatus in a Ti crucible. Bulk Ti samples (approximately 0.1 g each) and a Ti pellet were placed in the Ti crucible, as shown in Fig. 6(a). The Ti crucibles were placed with Ti sponge (getter) in a stainless-steel container, which was completely sealed via welding. The Ti samples, which were used for measuring O concentration, were cubes having 2–3 mm sides (Ti-2B, ca. 230 ppm O), 1.2 mm diameter wires (Ti-1.2U, ca. 1200 ppm O), and 2 mm diameter wires (Ti-2L, ca. 1200 ppm O). Ti pellets were made from hydride-dehydride Ti powder (press pressure: ∼500 MPa). Detailed information on the samples used in the experiments is shown in Table 3.

(a) Schematic illustration and (b) photograph (181001-lot.3-1) of the Ti crucible.

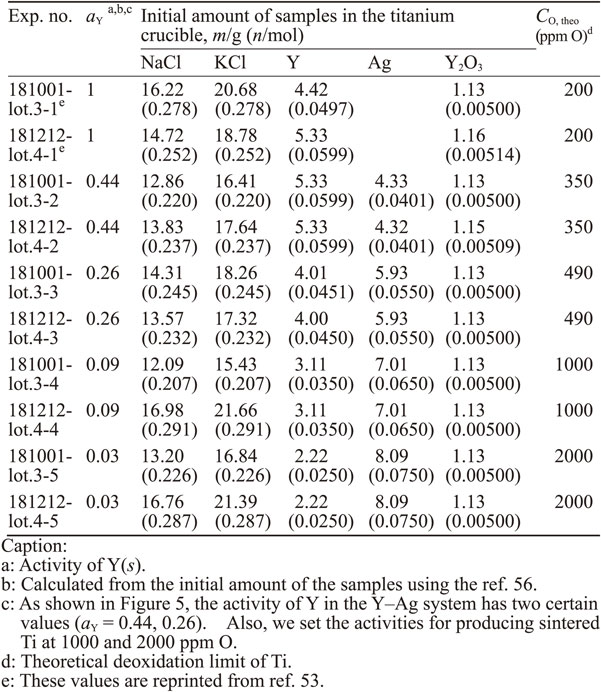
The initial amounts of samples and reagents in each lot are shown in Tables 4 and 5. In the experiments of the Y/Y2O3 equilibrium, approximately 1 g of Y2O3 was added into the initial feed to maintain $a_{\text{Y}_{2}\text{O}_{3}}$ at unity. The stainless-steel container was placed in a box-type electric furnace and held at 1300 K for 173 ks. The holding time was determined based on the diffusion coefficient of O in Ti at 1300 K58) and an assumption of the time required to obtain a homogeneous distribution of O dissolved in Ti.
After holding the stainless-steel container at 1300 K, the container was quenched in water. Figure 6(b) shows a cross-section of the Ti crucible after the experiment. The Ti sample for measuring O concentration was chemically polished with a 1:4:10 mixture of HF–HNO3–H2O, which was sufficient to remove impurities on the surface. It was then rinsed with distilled water and alcohol before drying. The O concentrations in the Ti sample was analyzed by inert gas fusion analysis (TC-600, LECO Corporation). Detailed analysis conditions are shown in Table 6.

After the experiment, salts attached to the Ti pellet were removed using acetic acid. Then, the surface of the Ti pellet was physically polished using a buffing grinder (# 120, # 1000, and # 2000) and chemically polished with HF–HNO3–H2O for approximately 30 s. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) were used to analyze the microstructure and the composition of the Ti pellet (JSM6510LV, JEOL Co., Ltd.).
Table 6 shows the O concentration in the Ti samples after the experiment in the Y/Y2O3 equilibrium. Figure 7 shows the relationship between the experimentally obtained O concentrations in Ti and aY in the Y–Ag alloy. As shown in Fig. 7, the results are in good agreement with calculated values using thermodynamics. These results indicate that the O concentration in Ti can be controlled reliably in the range of 200–2000 ppm O using the method designed in this study.
The relationship between experimentally obtained oxygen concentration in the Ti samples and aY in Y/Y2O3 equilibrium.
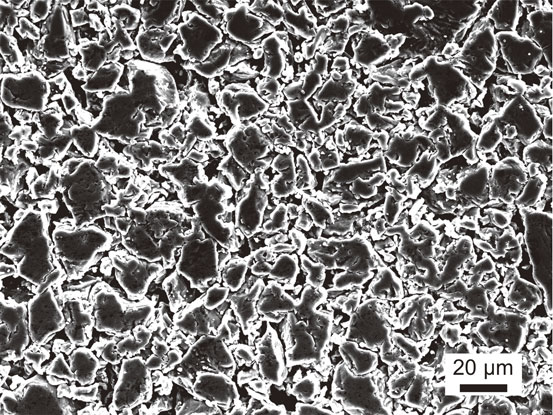
Figure 8 shows the SEM image of the surface of the Ti pellet before the experiment.
SEM image of the surface of Ti pellets in NaCl–KCl at 1300 K before the experiment.
Figure 9 shows the EDS mapping of a cross-section of a Ti pellet in the experiment in the Y/Y2O3 equilibrium, in which pure Y metal was employed in molten NaCl–KCl (Exp. # 181001-lot.3-1). Comparison of the SEM images in Figs. 8 and 9(a) indicates that sintering of Ti powder occurred. However, as shown in Fig. 9(a), the driving force for the sintering reaction of Ti powder was not high enough to obtain a completely dense sintered body. Furthermore, Y was observed in the Ti pellet (Fig. 9(e)), which means Y contamination was occurred in the Ti pellet. In contrast, the positions of the O and chlorine (Cl) signals in the Ti pellet were unclear (Figs. 9(b), (c)). Y contamination was likely caused from contact between the Ti powder and Y ions in the molten salt.

EDS-mapping of the cross-section of a Ti pellet in the Y/Y2O3 equilibrium, after the sintering experiment (exp.181001-lot.3-1): (a) SEM image, (b) O, (c) Cl, (d) Ti, and (e) Y.
Figure 10 shows the EDS mapping of a cross-section of a Ti pellet in the Y/YOCl/YCl3 equilibrium in YCl3 (Exp. #190315-lot.6-1). Figure 10 confirms that sintering occurred in this system and the sintered Ti pellet was still porous. The positions of Y, O, and Cl were not identified during the EDS mapping.
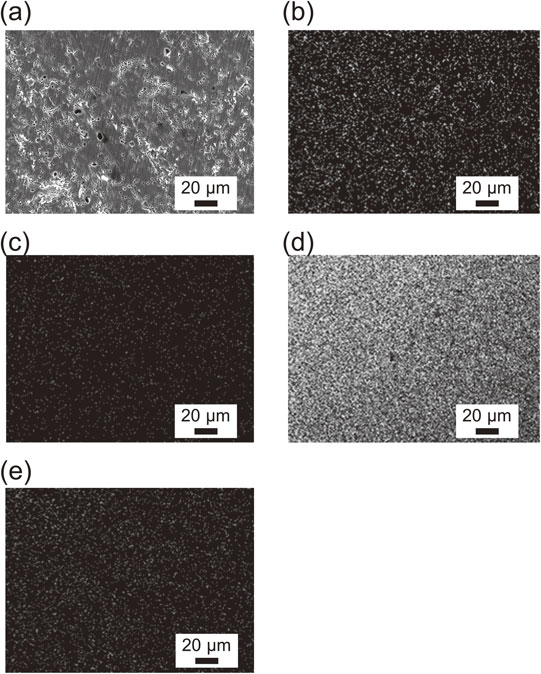
EDS-mapping of the cross-section of a Ti pellet in the Y/YOCl/YCl3 equilibrium, after the sintering experiment (exp.190315-lot.6-1): (a) SEM image, (b) O, (c) Cl, (d) Ti, and (e) Y.
In this study, we focused mainly on thermodynamics in order to probe the deoxidation limits during the sintering process. Thus, the temperature (1300 K) was set to be lower than the general sintering temperature of PM-Ti (1500–1700 K). However, the sintering/deoxidation temperature is changeable. Higher temperatures would increase the driving force of the sintering reaction under the ultra-low $p_{\text{O}_{2}}$ environment.
As mentioned, this study demonstrated that new deoxidation methods using the Y/Y2O3 and Y/YOCl/YCl3 equilibria are effective to control the O concentrations in Ti, and that the sintering reaction of Ti powder proceeds in molten salt at 1300 K. However, some problems, such as contamination and porousness in the sintered Ti, remain to be solved before this deoxidation method can be applied to PM-Ti in practice. The establishment of this new process will enable the efficient fabrication of Ti products with desired low-O-concentration from low-cost high-O-concentration Ti powder.
In this study, we developed a new sintering process that could remove O from Ti using Y metal in molten salt. This study showed that the O concentration in Ti could be reliably controlled by varying the activity of Y in the Y/Y2O3 equilibrium in NaCl–KCl (l) and that the sintering of Ti powder co-occurred. Additionally, when the Y/YOCl/YCl3 equilibrium was employed to reduce the O concentration in Ti to 30–60 ppm O in YCl3 (l), the sintering of Ti powder also proceeded. This study demonstrated the possibility of the development of a new sintering process, in which the O concentration of Ti can be controlled to 30–2000 ppm O.
We are grateful to Dr. Lingxin Kong and Mr. Takara Tanaka at The University of Tokyo for their helpful suggestions and help with the experiments. This work was financially supported by the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Scientific Research (S) (KAKENHI Grant No. 26220910, and 19H05623).