2021 年 62 巻 8 号 p. 1253-1262
2021 年 62 巻 8 号 p. 1253-1262
Disappearing of benthic seaweeds in coastal areas has gradually become a serious environmental problem worldwide. One possible reason is iron deficiency in seawater. As a major byproduct generated in steelmaking industry, steelmaking slag is rich in Fe and has the potential to restore seaweeds by supplying soluble Fe in seawater. The authors aim to develop a sustainable approach for ecosystem restoration in coastal areas by utilizing steelmaking slag. This work investigates the releasing behavior of elements from synthesized steelmaking slags in seawater and clarifies the effects of slag composition, carbonation and usage of gluconic acid. Slag with larger CaO/SiO2 ratio (= 3.0) has difficulty in releasing Fe into seawater directly, due to drastic increase of pH. Combination of slag carbonation and gluconic acid usage is necessary and effective in boosting release of Fe. Slag with smaller CaO/SiO2 ratio (= 1.0) would not cause drastic increase of pH in seawater and thus releasing of Fe is easier. These results have proved the potential value and feasibility of using steelmaking slag as an underwater iron fertilizer.

Benthic seaweeds are of great importance in marine ecosystem by offering food and shelter for thousands of associated sea animal and plant species.1) However, vanishing of seaweeds at barren ground in coastal areas has gradually become a serious problem in many countries.2,3) This unwanted phenomenon is probably attributed to multiple reasons, such as global warming, climate change and over-urbanization. One possible factor is deficiency of nutrient elements in neighboring seawater, especially iron (Fe).4) Suzuki et al. analyzed the seawater in the northern Japan sea with barren ground, and found that soluble Fe concentration is very low (<10.0 µg L−1).5) Fe ions function as a catalyst to activate the necessary enzymes when nutrients are ingested by the seaweeds. Matsunaga et al. clarified the relationship between soluble Fe and seaweeds growth by comparing the seaweeds growth rate in Fe-poor (0.0616 µg L−1) and Fe-rich (11.2 µg L−1) seawater.6) Faster growth of seaweeds in the Fe-rich medium was observed.
Steelmaking slag is a major byproduct generated in steelmaking industry. In 2019, the global output of steelmaking slag is estimated to be at least 190 million tons.7) Treatment of such a huge amount of solid waste is one of the challenges for the sustainable development of the industry. Steelmaking slag is normally reused in road construction and landfills as a concrete material. However, since valuable components are contained, it is possible to exploit new functions of steelmaking slag by utilizing these valuable components.
Containing Fe in the form of oxides, steelmaking slag is thought to be a potential underwater fertilizer for seaweeds. Natural leaching of steelmaking slag by seawater may release these nutrient elements which can be absorbed by seaweeds. Nakata et al.8) and Miki et al.9) studied the dissolution behaviors of hazardous elements (F, As, Pb, Cd, Cr6+, Hg etc.) from different types of steelmaking slags in seawater. Their results indicate that slag of carbon steel manufacturing process is safe because concentrations of hazardous elements in water are relatively low. Therefore, a rehabilitation technology of coastal area by natural leaching of steelmaking slag for nutrient elements has been proposed and practiced.10–15)
Utilization of steelmaking slag as an underwater fertilizer may simultaneously address the two challenges of seaweeds restoring and industrial byproduct treatment. Nakamura et al. investigated the seaweed growth in seawater enriched with steelmaking slag,10,12) and confirmed the positive effect of slag in supplying nutrient elements. Leaching behavior of elements from steelmaking slag under various conditions had been clarified.16–20) With the deepening of research, researchers gradually realized that direct usage of slag from basic oxygen furnace (BOF) in seawater is not the ideal, because this type of slag usually contains alkaline phases such as free CaO, which are reactive with water and would cause drastic increase in pH and precipitation of Mg as Mg(OH)2 (solubility product constant at 298 K: 5.61 × 10−12).21) On the other hand, ferrous ion [Fe (II)] released from slag is soluble yet easy to be oxidized to insoluble ferric ion [Fe (III)],22,23) which is not absorbable to seaweeds. Although bacteria can help to reduce ferric ion back to ferrous ion,24) a high concentration of bacteria in seawater is required. Therefore, pretreatment of BOF steelmaking slag and lifetime extension of soluble Fe ions in seawater are the difficulties to be addressed.
This work aims to find a solution to aid the releasing of Fe in seawater from steelmaking slag. It is thought that adjustment of slag composition, slag carbonation and addition of natural organic acid in seawater are simple approaches in operation. Carbonation can stabilize the alkaline components in the slag. Gluconic acid, being a typical natural organic compound normally found in humus, may extend the lifetime of soluble Fe by converting ferrous ion into a more stable form. As a fundamental study to exploit new functions of steelmaking slag, this work investigates the releasing behavior of elements from synthesized steelmaking slags in seawater under different conditions. The impact mechanisms of each factor are discussed. Based on the experimental results, a scheme for practical utilization of steelmaking slag in seawater is proposed.
Two slags with different composition were synthesized, carbonated and leached in artificial seawater with addition of gluconic acid. Releasing of major elements from slag into seawater was monitored by sampling at intervals and instant chemical analysis.
2.1 MaterialsMixture of synthesized CaO, FeOx, 3CaO·P2O5 and reagent grade SiO2 powders are melted in an iron crucible (inner diameter 30 mm, outer diameter 40 mm, height 140 mm) at 1723 K for 60 minutes in Ar atmosphere (flow rate: 1 L min−1), followed by quenching in blowing Ar and grinding to fine particles in air. Slag particles smaller than 150 µm were used in the experiment, although the size distribution was not measured. Chemical compositions of two synthesized slags as listed in Table 1 are plotted on the phase diagram of the CaO–FeO–(SiO2 + P2O5) pseudo ternary system in Fig. 1. The grey zone indicates the liquid region for the CaO–FeO–SiO2 system equilibrated with iron at 1723 K.25) The red zone indicates the approximate composition range of actual BOF slags. Slag-I with larger CaO/SiO2 ratio (= 3.0), located in the 2CaO·SiO2-saturated region at steel refining temperature (approximately 1723 K), is designed to represent the BOF slag. Slag-II with smaller CaO/SiO2 ratio (= 1.0), located in the liquid region, is used to represent the composition-adjusted BOF slag.


Slag compositions plotted on the CaO–FeO–(SiO2 + P2O5) pseudo phase diagram.
The artificial seawater was prepared by dissolving 950 g of sea salt (Marine art SF-1, Tomita Pharmaceutical Co. Ltd.) into 12.5 L of distilled water, concentrations of major constituents of which are shown in Table 2. Gluconic acid solution were prepared by diluting gluconic acid (Analytical reagent grade, concentration: from 47.0 to 53.0 mass%, density: 1.24 g cm−3, Wako Pure Chemical Industries Ltd.) with artificial seawater. The pH value was adjusted to be the same with seawater (pH = 8.2) by adding NaOH (aq, 0.1 mol L−1) or HCl (aq, 0.1 mol L−1).

As described in a previous paper,26) carbonation was performed in a quartz column (inner diameter 46 mm, height 140 mm) filled with slag (approximate 30 g) and distilled water (approximate 3 ml) at room temperature and atmospheric pressure. CO2 (flow rate: 135 ml min−1) saturated with water vapor flowed through the column from the bottom. The column was weighed in every 10 minutes. CO2 was introduced continuously until the column showed no gain in weight. Afterwards the slag was dried at 423 K for 10 hours in air. This procedure was conducted twice to achieve higher carbonation ratio. Crystalline phases in slag were characterized by powder X-ray diffraction (Smart lab, Rigaku Corp.). Carbon contents were analyzed by the carbon combustion method (CSLS-600, LECO Corp.).
2.3 LeachingBatch mode leaching experiments were conducted at 298 K, also as described in a previous paper.26) A polypropylene bottle (outer diameter 63 mm, height 120 mm, volume 250 ml) containing 1 g of slag and 100 ml of artificial seawater (with or without addition of gluconic acid) was shaken at a speed of 160 cycles/min for up to 192 h. Sampling was conducted at intervals. The pH of the solution sample were immediately measured by a pH meter (IM-55G with GST-5731C probe, DKK-TOA Corp.). After removal of possible solid residues by passing through an Omnipore membrane filter (pore size 0.45 µm, Merck Millipore), chemical analysis of the solution were conducted. Concentrations of Ca, Mg and Si were analyzed by an inductively coupled plasma optical emission spectrometer (ICP-OES, SPS7800, Seiko Instruments Inc.). Total Fe was analyzed by an inductively coupled plasma mass spectrometer (ICP-MS, SPQ9000, Seiko Instruments Inc.). P was analyzed by the molybdenum blue spectrometric method (U-2001 spectrometer, Hitachi Ltd.).
Figure 2 shows the XRD patterns of two synthesized slags before and after carbonation. Both slags mainly contain crystal phases, although glassy phases may also present. For slag-I, apparent change could be noticed. 2CaO·Fe2O3 and 2CaO·SiO2 are detected before carbonation, while CaCO3 is newly observed after carbonation. Fe3+ is likely to be formed due to oxidation when grinding the slag into fine particles in air. For slag-II, XRD pattern shows little dependence on carbonation. CaO·2FeO·SiO2 is detected before and after carbonation. According to carbon analysis, contents of carbon after carbonation are 1.93 mass% and 0.05 mass% for slag-I and slag-II, respectively. These results indicate that CaO·2FeO·SiO2 is not reactive with CO2, while 2CaO·Fe2O3 and 2CaO·SiO2 are easy to react with CO2 by
| \begin{equation} \text{2CaO${\cdot}$Fe$_{2}$O$_{3}$ (s)} + \text{2CO$_{2}$ (g)} = \text{2CaCO$_{3}$ (s)} + \text{Fe$_{2}$O$_{3}$ (s)} \end{equation} | (1) |
| \begin{equation*} \Delta G_{\text{(1), 298K}}^{\text{o}} = -209.5\,\text{kJ}\,\text{mol$^{-1}$}\,^{27)} \end{equation*} |
| \begin{equation} \text{2CaO${\cdot}$SiO$_{2}$ (s)} + \text{2CO$_{2}$ (g)} = \text{2CaCO$_{3}$ (s)} + \text{SiO$_{2}$ (s)} \end{equation} | (2) |
| \begin{equation*} \Delta G_{\text{(2), 298K}}^{\text{o}} = -126.7\,\text{kJ}\,\text{mol$^{-1}$}\,^{27)} \end{equation*} |

XRD patterns of two synthesized slags before and after carbonation.
Figure 3 shows the XRD patterns of slags after leaching for 192 h in seawater with or without gluconic acid 0.5 g L−1. As compared with the XRD patterns before leaching in Fig. 2, little difference could be noticed for each case, except for the slight decrease of peak intensity. Surface conditions of the slags may have changed. In general terms, it is believed that leaching of slags in seawater would not cause apparent phase change.

XRD patterns of (a) slag-I and (b) slag-II (non-carbonated and carbonated) after leaching in artificial seawater (containing 0 and 0.5 g L−1 of gluconic acid) for 192 h at 298 K.
Concentration of Fe in initial sweater is extremely low (1.5 µg L−1). Figure 4 shows the variation of concentration of Fe during slag leaching in seawater with addition of different amount of gluconic acid. When using both slags in seawater without gluconic acid, release of Fe is negligible little and carbonation has no effect [Fig. 4(a)]. For slag-I, slag carbonation and gluconic acid usage in combination is effective in boosting Fe release. When using carbonated slag-I in seawater containing gluconic acid 0.500 g L−1, the maximum concentration of Fe increases to 3.5 mg L−1 [Fig. 4(d)], more than 2000 times as large as the value in the initial seawater.

Variation of concentration of Fe in artificial seawater during slag leaching at 298 K. (a) without gluconic acid, (b) gluconic acid 0.125 g L−1, (c) gluconic acid 0.250 g L−1, and (d) gluconic acid 0.500 g L−1. Slag:seawater = 1:100 in weight.
For slag-II, addition of gluconic acid improves release of Fe, regardless of carbonation. When using slag-II in seawater containing gluconic acid 0.500 g L−1, the maximum concentration of Fe reaches 7.5 mg L−1. Carbonation shows a negative effect on release of Fe from slag-II.
3.3 Variation of P in seawaterPhosphorus is not contained in the initial seawater. Figure 5 shows the variation of concentration of P in seawater with addition of different amount of gluconic acid. For slag-I, release of P is possible only for carbonated slags, and the effect of gluconic acid usage is not noticeable.

Variation of concentration of P in artificial seawater during slag leaching at 298 K. (a) without gluconic acid, (b) gluconic acid 0.125 g L−1, (c) gluconic acid 0.250 g L−1, and (d) gluconic acid 0.500 g L−1. Slag:seawater = 1:100 in weight.
For non-carbonated slag-II, a gradual increase of P in seawater with leaching time can be observed, indicating the release of P from slag. Addition of gluconic acid shows a positive effect. The maximum concentration of P increases to 0.9 mg L−1 [Fig. 5(d)]. Similar to its effect on Fe releasing, carbonation also shows a negative effect on release of P from slag-II.
3.4 Variation of pH of seawaterFigure 6 shows the variation of pH of seawater with addition of different amount of gluconic acid. Leaching slag-I in seawater results in immediate increase of pH from the initial value of 8.2. Increase of pH is due to hydration of alkaline mineral phases such as 2CaO·SiO2 in slag. According to the literature, the following reactions are considered:
| \begin{align} &\text{2CaO${\cdot}$SiO$_{2}$(s)} + \text{4H$_{2}$O(aq)} \\ &\quad = \text{3CaO${\cdot}$2SiO$_{2}{\cdot}$3H$_{2}$O(s)} + \text{Ca(OH)$_{2}$(aq)}^{28)} \end{align} | (3) |
| \begin{align} &\text{2CaO${\cdot}$SiO$_{2}$(s)} + \text{2.61H$_{2}$O(aq)} \\ &\quad = \text{1.8CaO${\cdot}$SiO$_{2}{\cdot}$2.41H$_{2}$O(s)} + \text{0.2Ca(OH)$_{2}$(aq)}^{29)} \end{align} | (4) |
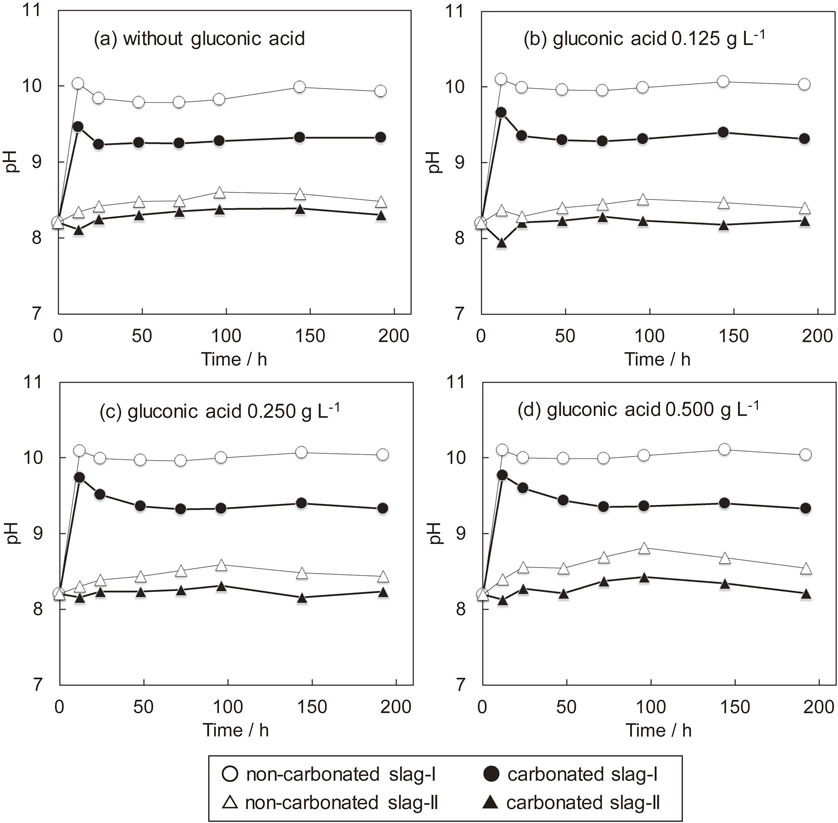
Variation of pH of artificial seawater during slag leaching at 298 K. (a) without gluconic acid, (b) gluconic acid 0.125 g L−1, (c) gluconic acid 0.250 g L−1, and (d) gluconic acid 0.500 g L−1. Slag:seawater = 1:100 in weight.
For non-carbonated slag-II, an increasing tendency of pH is also observed, yet to a lesser extent compared with that of slag-I due to containing less amount of alkaline mineral phases. The result indicates that adjusting the slag composition to a smaller CaO/SiO2 ratio is effective in avoiding the sharp increase of pH. When using carbonated slag-II, pH is almost constant, regardless of leaching time and addition of gluconic acid.
3.5 Variation of Ca in seawaterFigure 7 shows the variation of concentration of Ca in seawater with addition of different amount of gluconic acid. For non-carbonated slag-I, concentration of Ca increases apparently. Addition of gluconic acid leads to a further slight increase. When using carbonated slag-I, increase of Ca is apparently alleviated.

Variation of concentration of Ca in artificial seawater during slag leaching at 298 K. (a) without gluconic acid, (b) gluconic acid 0.125 g L−1, (c) gluconic acid 0.250 g L−1, and (d) gluconic acid 0.500 g L−1. Slag:seawater = 1:100 in weight.
When using slag-II, concentration of Ca in seawater is almost constant, and independent of carbonation or addition of gluconic acid.
3.6 Variation of Mg in seawaterFigure 8 shows the variation of concentration of Mg in seawater with addition of different amount of gluconic acid. Mg suffers a remarkable decrease when using slag-I. The decrease is even worse with addition of gluconic acid. By using carbonated slag-I, decrease of Mg is obviously alleviated.

Variation of concentration of Mg during leaching slags in artificial seawater at 298 K. (a) without gluconic acid, (b) gluconic acid 0.125 g L−1, (c) gluconic acid 0.250 g L−1, and (d) gluconic acid 0.500 g L−1. Slag:seawater = 1:100 in weight.
When using slag-II, concentration of Mg in seawater is almost constant. Slag carbonation or addition of gluconic acid exerts little effect in Mg variation.
The results indicate that supplying Fe from steelmaking slag into seawater is possible, especially when using slag-II. An increase of several thousand times in concentration of Fe is expected. Figure 9 plots the variation of average concentration of Fe in seawater after slag leaching for 96, 144 and 192 h under different conditions. Although both slags contain the same amount of FeO, leaching behaviors of Fe from two slags into seawater are very different, and are strongly influenced by slag carbonation and usage of gluconic acid. Release of Fe is easier from slag-II compared with that from slag-I.

Dependences of concentration of Fe in seawater after leaching (a) slag-I and (b) slag-II on carbonation and usage of gluconic acid in seawater at 298 K. Average concentration of Fe in seawater after leaching for 96, 144 and 192 h are plotted.
Figure 9 indicates that usage of gluconic acid is necessary for a marked release of Fe regardless of slag composition. Slag hydration may generate Fe(OH)2, Fe(OH)3 or FeOOH, which are probably the solid substances to release Fe into seawater. Because solubility of Fe(II) is much larger than that of Fe(III) in water at 298 K,30) FeOH+ is considered as the dominant soluble species in seawater due to the reaction
| \begin{equation} \text{Fe(OH)$_{2}$(s)} + \text{H$^{+}$} = \text{FeOH$^{+}$} + \text{H$_{2}$O} \end{equation} | (5) |
| \begin{equation} \text{FeOH$^{+}$} + \text{2H$_{2}$O} = \text{Fe(OH)$_{3}$(s)} + \text{2H$^{+}$} + \text{e$^{-}$} \end{equation} | (6) |
| \begin{equation*} E_{(6)} = E_{(6)}^{0} + \frac{2.303RT}{F}\log\frac{[\text{H$^{+}$}]^{2}}{[\text{FeOH$^{+}$}]}, \end{equation*} |
| \begin{equation} E_{(6)} = 0.5451 - 0.1183\,\text{pH} - 0.0592\log C_{\text{Fe}} \end{equation} | (7) |


Potential-pH diagram of Fe–H2O system considering Fe(OH)3 as the solid substance over pH range between 7 and 11 at 298 K. Measured redox potentials when leaching slags in seawater without or with gluconic acid 0.500 g L−1 are plotted on the diagram.
When seawater contains gluconic acid (HGH4), Fe-based chelated complex would be formed31,32) from FeOH+ through
| \begin{equation} \text{HGH$_{4}$}\rightleftharpoons \text{H$^{+}$} + \text{GH$_{4}^{-}$} \end{equation} | (8) |
| \begin{equation} \text{FeOH$^{+}$} + \text{H$^{+}$} = \text{Fe$^{2+}$} + \text{H$_{2}$O} \end{equation} | (9) |
| \begin{equation} \text{Fe$^{2+}$} + \text{GH$_{4}^{-}$} = \text{Fe(GH$_{4}$)$^{+}$} \end{equation} | (10) |
CaO/SiO2 ratio of the slag is considered to be the major factor which determines the release behavior of Fe. For slag-I with a larger CaO/SiO2 ratio (= 3.0), 2CaO·SiO2 and 2CaO·Fe2O3 phases may react with water to form Ca(OH)2, leading to a sharp increase in pH (Fig. 6), which invalidates gluconic acid. Therefore, in order to use slag-I as a nutrient supplier, deployment of slag carbonation and usage of gluconic acid in combination is necessary. Slag carbonation alleviates the sharp increase of pH, while usage of gluconic acid extends the lifetime of soluble Fe. For slag-II with a smaller CaO/SiO2 ratio (= 1.0), change in pH of seawater is not remarkable and thus carbonation is not necessary. Negative effect of carbonation on Fe release from slag-II may due to the change of slag surface structure induced by carbonation.
Since an Ar quenching method was employed to prepare the slag samples, glassy phases may present in both slags. Release of Fe from instable glassy phases is expected to be easier compared with crystal phases which are more stable. The type of Fe-containing crystalline phase should also influence the release of Fe, due to the different lattice energy for different phase. These issues will be clarified in a future study.
4.2 Releasing of P from slag in seawaterLike the case of Fe, release of P is also easier from slag-II compared with that from slag-I. Figure 11 shows the variation of average concentration of P in seawater after slag leaching for 96, 144 and 192 h under different conditions. Slag-II can be used directly to supply P in seawater, while slag-I must be carbonated before usage.

Dependences of concentration of P in seawater after leaching (a) slag-I and (b) slag-II on carbonation and usage of gluconic acid in seawater at 298 K. Average concentration of P in seawater after leaching for 96, 144 and 192 h are plotted.
Since P2O5 in steelmaking slag tends to enrich in 2CaO·SiO2 phase to form 2CaO·SiO2–3CaO·P2O5 solid solutions during refining or solidification, 3CaO·P2O5 is considered as the major solid substance to release HPO42−, which is the stable state, in the present slag–seawater system. The reaction could be expressed as
| \begin{equation} \text{3CaO${\cdot}$P$_{2}$O$_{5}$(s)} + \text{2H$^{+}$} = \text{3Ca$^{2+}$} + \text{2HPO$_{4}^{2-}$} \end{equation} | (11) |
| \begin{equation*} K_{(11)} = \frac{[\text{Ca$^{2+}$}]^{3}[\text{HPO$_{4}^{2-}$}]^{2}}{[\text{H$^{+}$}]\,^{2}} \end{equation*} |
Mg is one of the most important elements in the marine biological system. This work indicates that the problem of Mg loss can be avoided by rational usage of steelmaking slag such as carbonation or adjusting the slag composition to a smaller CaO/SiO2 ratio.
The pH of seawater determines the solubility of Mg due to the reaction
| \begin{equation} \text{Mg$^{2+}$} + \text{2H$_{2}$O} = \text{Mg(OH)$_{2}$ (s)} + \text{2H$^{+}$}. \end{equation} | (12) |
| \begin{equation*} K_{(12)} = \frac{[\text{H$^{+}$}]^{2}}{[\text{Mg$^{2+}$}]} = \frac{[\text{H$^{+}$}]^{2}}{C_{\text{Mg${^{2+}}$}}\gamma_{\text{Mg${^{2+}}$}}} \end{equation*} |
| \begin{equation} \log(C_{\text{Mg${^{2+}}$}}\gamma_{\text{Mg${^{2+}}$}}) = 16.9 - 2.0\,\text{pH}. \end{equation} | (13) |
Since Platford33) had measured that $\gamma _{\text{Mg}^{2 + }}$ is around 0.063 in seawater at 298 K, dependence of equilibrium concentration of Mg2+ on pH at 298 K could be expressed by
| \begin{equation} \log C_{\text{Mg${^{2+}}$}} = 18.1 - 2.0\,\text{pH} \end{equation} | (14) |

Solubility diagram of Mg(OH)2 in water at 298 K. Experimental results are also plotted on the diagram.
The leaching experiment in this work confirms the potential value of steelmaking slag as an underwater fertilizer. Although direct usage of slag with large CaO/SiO2 ratio may cause pH increase and Mg loss while supply of Fe is limited, the disadvantages can be addressed by approaches such as adjusting the slag composition, carbonation or using organic acids such as gluconic acid. Considering the application in practice, organic acids can be provided by using natural substances such as humus, compost or soil. Therefore, we believe that mixing humus, compost or soil with composition-adjusted or carbonated slag is an efficient and economical approach. The mixture can be made into artificial fertilizers, to be sunk in marine coastal area as a supplier of Fe. Growth of seaweeds surrounding these artificial fertilizers is expected.
As a fundamental study to develop a new technology of utilizing steelmaking slag to fertilize coastal seaweeds, this work investigates the releasing behavior of elements from steelmaking slag in seawater. The experiment of leaching two synthesized steelmaking slags in artificial water is conducted. The effects of slag composition, carbonation and usage of gluconic acid on change of seawater composition are clarified.
Slag-I with larger CaO/SiO2 ratio (= 3.0) has difficulty in releasing Fe into seawater directly and causes problem of Mg loss, due to drastic increase of pH. Combination of slag carbonation and gluconic acid usage is necessary and effective in boosting release of Fe while avoid Mg loss. Slag-II with smaller CaO/SiO2 ratio (= 1.0) would not cause drastic increase of pH and Mg loss in seawater. Usage of gluconic acid is still needed to improve the release of Fe.
These results have proved the potential value and feasibility of using steelmaking slag as an underwater fertilizer. Although direct usage of slag may be inappropriate, the disadvantages can be addressed by approaches such as adjusting the slag composition, carbonation or using gluconic acid.