2022 年 63 巻 2 号 p. 128-132
2022 年 63 巻 2 号 p. 128-132
The physical properties desired for various applications of aluminum alloys are usually modified by tailoring their precipitation behavior during heat treatment. In this study, the precipitation behavior of a cast Al–Mn alloy annealed with two heating methods (electric resistance heating and radiative furnace heating) at different temperature has been studied through the electrical conductivity (EC) test, and scanning electron microscope. The impact of the two heating methods on the mechanical properties of the treated Al–Mn samples was evaluated through hardness and EC analysis. The results clearly show that under the conditions of electric resistance heating, the precipitation kinetics of the alloy is promoted. The mechanisms behind this phenomenon are discussed in detail.
AA3xxx series aluminum alloys are widely used in metal packaging and auto parts industries due to their moderate strength, good ductility, and high processability. The main alloying element is Mn, but Fe, Si, Cu, Mg and other elements are also added depending on the intended applications. During the heat treatment, supersaturated Mn in the as-cast material will precipitate to form second-phase particles. The equilibrium precipitation phase of AA3xxx is Al6Mn,1) body-centered cubes G (Al12Mn) and orthogonal phase (Al7Mn) can also exist at low temperatures. It is well known that the number, size, and spatial distribution of precipitates greatly affects the physical properties of the alloy.2–4) However, the presence of manganese, which has very low diffusion rate in Al matrix, makes natural aging of the alloy extremely slow at room temperature.5) Even though AA3xxx alloys are typically non-heat treatable, the strengthening effect form the precipitated dispersoids was well documented in the literature.6,7) Therefore, the dispersoids strengthening through heat treatment process is actually also very important for the practical application of AA3xxx series aluminum alloys.
During heat treatment, different heating methods are used, including traditional radiant heating,8) salt bath heating,8) induction heating,9) and electric resistance heating (or electric current heating),10) Among them, electric resistance heating was first proposed by Troitskii and his colleagues.11) The use of the Joule effect greatly improves the heating efficiency, and it is currently widely studied. Electric resistance heating has been researched and applied in steel,12) Al,10,13–16) Zr,17) Ti,18,19) Cu,20,21) Ni22) and other alloys. Heat treatment with electric resistance heating has been demonstrated to affect both recrystallization and precipitation behavior of these alloys.
Most of the current studies have shown that electric current promotes the occurrence of recrystallization. Earlier study on the effect of electric current on recrystallization of cold-worked α-Ti showed that electric current promoted nucleation of recrystallization.19) Relying on vacancy concentration and grain boundary expansion, continuous current significantly accelerates the recovery and recrystallization of pure copper.20) Our previous work demonstrated that continuous alternation electric current accelerated recrystallization kinetics, and this effect depends on the prior cold-deformation and the relative direction of the applied current.10) Furthermore, Liang et al.21) even found that electric current stressing induced the recrystallization of non-deformed brass due to Joule heat combined with high-density dislocations produced by electromigration force. In addition, the pronounced acceleration of the kinetics of dynamic recrystallization by Joule effect heating has been confirmed in Inconel 718 superalloy.22)
But for particle-containing materials, current studies have shown that electric current treatment has a double-edge sword effect on precipitation. On the one hand, the electric current induces lattice strain and subsequent dissolution of the second-phase precipitate in the alloy matrix.23,24) On the other hand, the diffusion of alloying elements is accelerated by the current, which promotes the nucleation of precipitates, increases the number of second-phase precipitates, and also promotes the growth of precipitates.13,25,26) Current can both increase the precipitation rate and delay the precipitation rate, depending on the alloy, current density and frequency.25) The influence mechanism of electromigration induced by current in the precipitation process is still unclear.
Further, most of the above studies have focused on the qualitative changes brought about by electric current heating compared to traditional radiative furnace heating. The temperature-time path of the two heating methods is usually not exactly the same, which makes it impossible to quantitatively clarify the real difference between electric resistance heating and traditional heating methods. Meanwhile, the complex interaction between recrystallization and precipitation was mostly neglected even for particle-containing alloys. Therefore, in this work, traditional radiative furnace heating and electric resistance heating were performed under the identical temperature-time path, in order to study the influence of the electric current on precipitation behavior of a cast AA3xxx aluminum where recrystallization is absent during heat treatment. The correlation between the different precipitation states and mechanical properties was also discussed in detail.
The as-received material is a direct-chilling cast extrusion billet, with chemical composition of: Mn 0.39 mass%, Si 0.15 mass%, Fe 0.5 mass% and the balance of Al. On one hand, a very small sample with size of Φ10 × 8 mm, to ensure temperature homogeneity, was welded with a K-type thermocouple to obtain the temperature-time curve during radiative furnace heating. Subsequently, this small sample was inserted from a small hole into the traditional heating furnace for radiative heating at 400°C, 450°C, and 500°C, without opening the furnace. Since the samples were hanging in the air of the furnace chamber thanks to the thermocouple lines, there was actually no conductive heating. The holding time was 100 s, 500 s, 1000 s and 5000 s, respectively. On the other hand, the as-received material was processed by wire cutting to get cylindrical samples with same size (Φ10 × 8 mm) for electric current heating on a Gleeble 3800 machine. Because Al has good thermal conductivity, it is easy to obtain a large amount of uniform temperature through the Joule effect annealing (using electric current), especially when heating is conducted under vacuum conditions. The temperature-time curve collected from radiative furnace heating was then imported into Gleeble 3800 machine. Since the heating rate is relatively slow, especially when approaching the target temperature, it can ensure that the same temperature-time was applied to both radiative furnace heating and electric resistance heating.
2.2 Microstructure and properties characterizationThe precipitation behavior during heat treatment of the samples was first indirectly investigated by Vickers hardness (VHN) and electrical conductivity (EC) measurements, respectively. Eight measurements were conducted on the samples for both VHN and EC, from which their average values and standard deviations were derived. For the VHN, the measurements were performed using a load of 200 g, a loading speed of 100 µm/s and a loading time of 15 s. Since no deformation was applied, recrystallization will not take place during the heat treatment with different heating methods. The VHN value in this study is mainly used to reflect the change of mechanical properties, considering that it is difficult to further machine tensile test sample from the small heat treated samples (Φ10 × 8 mm). EC measurements were performed at room temperature with a Sigma 2008 device. From the literature,1) it is known that the precipitated phase is Mn-containing particles. The precipitation will change the conductivity of the alloy, since it reduces the lattice distortion induced by supersaturation of alloying elements. Therefore, the variation of EC can be linked to the change of Mn content in the alloy matrix. Since the precipitation of Mn-containing particles within the aluminum matrix increases its EC value, it can be used as a semi-quantitative way to estimate the precipitation behavior of Mn-containing particles. This not only indirectly indicate the amount of precipitation in large areas but also avoid tedious metallographic inspection. Subsequently, the area near the welded thermocouple of the sample was directly characterized in terms of precipitation number and size. First, mechanical polishing was carried out according to standard metallographic procedures,27) and then electrolytic polishing was carried out using LectroPol-5. Finally, the grain structure and second-phase precipitates were observed by a GEMINI 500 scanning electron microscope (SEM) with electron backscattered diffraction (EBSD).
The morphology and distribution of constituent particles can be seen in Fig. 1(a), and no fine second-phase particle is observed in the as-received alloy (Fig. 1(b)), which was well documented in the literature for this type of material.27) After two kinds of heating treatment, we obtained the grain structure at different temperature and different time through EBSD, as shown in Fig. 2. It is clear that the original grain structure was kept regardless of the heating methods, heating temperature and heating time. This implies that grain structure evolution can be ignored when analyzing the effect of heating method on precipitation behaviour.

Initial microstructure of Al–Mn alloy: (a) low magnification and (b) high magnification microstructure.

IPF map of different heat treated samples: the samples were kept at (a) 400°C and (b) 450°C for 5000 s respectively, heat to 500°C for (c) 500 s and (d) 5000 s. (a)–(d) were heated by radiative furnace. The samples were heated to (e) 400°C and (f) 500°C by electric resistance and kept for 5000 s.
Since no grain structure evolution (e.g. recrystallization) was found during heat treatment, focus can then be shifted to the precipitation behavior which will be first indirectly examined by the variation of VHH and EC data. It can be seen from Fig. 3(a) that EC increases with annealing time at different annealing temperatures for both heating methods. The samples heated by electric resistance heating always have higher EC than their counterparts treated by radiative furnace heating. Annealing at higher temperatures promote the precipitation of dispersoids, sluggish precipitation is observed for the sample treated by radiative furnace heating at 400°C. Similar trend can be found for the variation of hardness in that longer annealing time leads to larger VHN values, annealing by electric resistance heating also yields higher VHN values, as illustrated in Fig. 3(b). However, increasing the annealing temperature from 450°C to 500°C actually leads to a decrease in VHN when the samples were annealed with electric current. It should also be noted that both EC and VHN have not reached to the steady state yet, which facilitates the comparison of different heating methods on precipitation behavior.
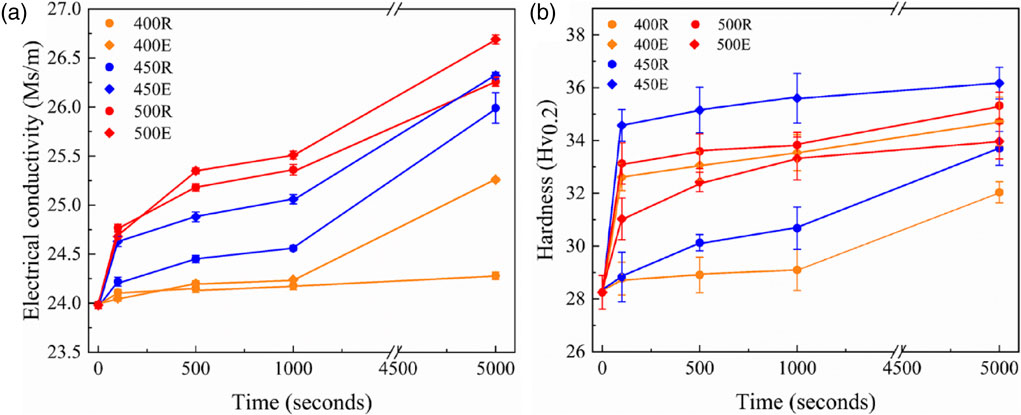
Variety in the (a) electrical conductivity and (b) hardness of the alloy after different heat treatments. 400R represents radiative furnace heating to 400°C, 400E represents electric resistance heating to 400°C.
It is known that electric current also affects recrystallization behavior,28) and the complex interaction between recrystallization and precipitation will make the analysis more difficult. Since the starting material is in as-cast state without deformation, the driving force for recrystallization is too low to trigger recrystallization under the different heat treatment conditions, the interaction between recrystallization and precipitation can thus be avoided in this study. The true effect of electric current on precipitation of AA3xxx alloys can be more precisely analyzed.
For the second-phase particles, it is well known that their precipitation can be divided into nucleation, growth and coarsening stages. The diffusion of manganese in the matrix plays a key role in the precipitation behavior of 3xxx alloys and the evolution of the dispersoids size due to the extremely high manganese content in the precipitates.1) At low temperatures, the dispersoids can only be nucleated in local areas with high manganese content due to the low diffusion rate. As the temperature increases, manganese precipitates out of the supersaturated solid solution, which can be seen indirectly in Fig. 3(a). The increase in EC is the result of continuous decomposition of solid solution.1) In the two heating methods, the conductivity increases with the increase in temperature and holding time. This is related to the precipitation mainly controlled by nucleation and growth.1) At the same time, it can be seen from Fig. 3(a) that the EC of the sample heated by electric current is higher than that treated by radiative furnace heating at all temperatures. This seems to indicate that heating the sample with electric current promotes nucleation of precipitation, due to the electron transfer effect of the current.1) Admittedly, the electric field associated with the current may also affect the barrier that must be overcome when the solute atoms detached from the matrix are diffused to the precipitate.25) Meanwhile, it can be seen from Fig. 3(b) that with the increase of precipitation, the hardness of the material increases. When the samples were annealed by radiative furnace heating, the hardness also increase with temperature. However, this is not the case for the sample treated by electric current where the hardness is the highest at 450°C (see the blue line with diamond symbol in Fig. 3(b)). This is most likely because the number of second-phase particles at this temperature is more than that at 400°C, while their average size is smaller than that at 500°C. The combination of these two factors results in, the maximum hardness at 450°C. This explanation seems reasonable but requires more direct evidence.
The second-phase particles at high temperatures were directly characterized by SEM, as shown in Fig. 4. For radiative furnace heating, it can clearly be seen that the number of precipitates increase with temperature (from 450°C to 500°C) while their average size (∼58 nm) is more or less unchanged. It is well known that more fine precipitates contribute to higher strength. This agrees well with the variation of hardness shown in Fig. 3(b), i.e., the hardness of samples after radiative furnace heating increases with temperature. Regarding the samples heated by electric resistance, it can be seen in Fig. 3(a) that the conductivity of the electric resistance heated samples are higher than that treated by radiative furnace heating under the same temperature and time, indicating that the current can effectively increase the precipitation rate.25) The EC value does still increase when heated at 500°C, but the supersaturated Mn mainly contribute to the dissolution of fine precipitates and coarsening of existing precipitates (see Fig. 4(c)) instead of forming new fine precipitates. The coarsening of precipitates leads to the decrease of hardness for the samples subjected to electric resistance heating, as illustrated in Fig. 3(b).
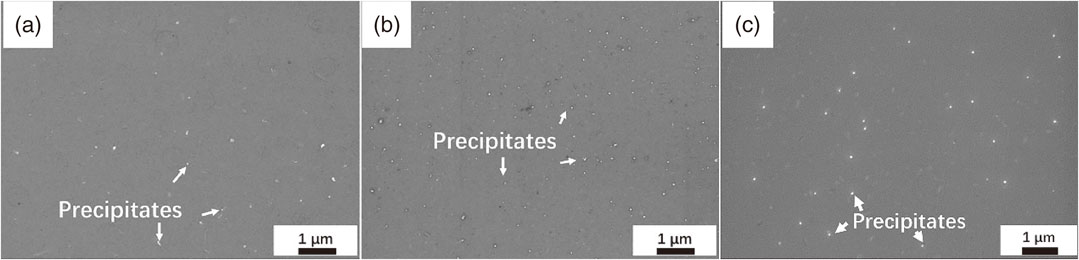
SEM images of precipitated phases after radiation heating to different temperatures for 5000 s: (a) 450°C, (b) 500°C. And (c) electric heating to 500°C for 5000 s.
At low temperature of 400°C, where precipitates coarsening rate is pretty small due to the low diffusion rate, the higher EC value of electric resistance heated samples should be ascribed to the faster nucleation rate of the precipitates. This can also be confirmed by its higher hardness value as compared to its counterparts subjected to radiative furnace heating, as shown in Fig. 3(b). At high temperature of 500°C, on the other hand, the electric current promotes coarsening of large precipitates and dissolution of fine precipitates. The acceleration of nucleation of precipitation at low temperature, as well as the dissolution of fine precipitates and coarsening of large precipitates at high temperature may all be explained by the effect of electric current on the diffusion of atoms due to electromigration. It has been documented that the influence of current on precipitation nucleation and precipitate growth varies with heat treatment temperature.13) That’s to say, the effect of electric current on precipitation behavior, i.e. nucleation, coarsening and dissolution, depends on the phase transformation temperature of the precipitates, which is mainly determined by the chemical composition of the material.
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China [51805415], the Natural Science Basis Research Plan in Shaanxi Province of China [Program No. 2019JM-125] and the Open Research Fund of State Key Laboratory of High Performance Complex Manufacturing, Central South University [Kfkt2018-04]. We thank Mr Zijun Ren at Instrument Analysis Center of Xi’an Jiaotong University for his assistance with scanning electron microscope analysis.