2023 年 64 巻 1 号 p. 242-251
2023 年 64 巻 1 号 p. 242-251
Cu electrorefining using a low-grade copper anode is desirable from the standpoint of electric power savings. Cu electrolysis was carried out in an unagitated sulfate solution with a low-grade copper anode, and the effect of impurity ions and additives in the solution on the passivation of the anode was investigated. The time when anode passivation firstly occurs shortened significantly in a solution containing 0.596 mol·dm−3 of Ni2+ ions as impurity and shortened somewhat in a solution containing As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions. Sn2+(0.0004 mol·dm−3) and As3+(0.053 mol·dm−3) ions slightly decreased the time to passivation, but Sb3+(0.004 mol·dm−3) ions did not. The viscosity coefficient of the solution increased when the 0.596 mol·dm−3 of Ni2+ ions were added to the solution, while the diffusion coefficient of Cu2+ ions decreased. The compound of the As–Sb–O or As–Bi–O system was formed in anode slime when the As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions were added to the solution, which seemed to increase the compactness of slime. The time to passivation was slightly longer in thiourea-free solution but shortened when the concentration of thiourea was increased from 0.525 to 2.24 mmol·dm−3. The time to passivation was constant in solutions containing 0 to 1.13 mmol·dm−3 of Cl− ions, but significantly decreased as the concentration of Cl− ions increased above 1.13 mmol·dm−3. Cl− ions formed Cu–Cl at the upper area of anode slime, which increased the compactness of slime and promoted the passivation.
This Paper was Originally Published in Japanese in J. Japan Inst. Metals 86 (2022) 97–106.
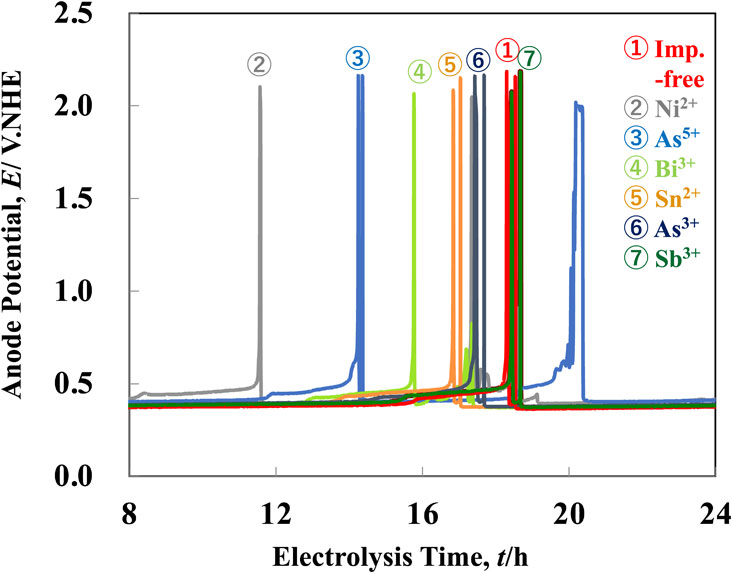
Fig. 2 Time dependence of anode potential during electrolysis at 200 A·m−2 in the solutions containing various kinds of impurities. [Additive: Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3), Impurity: Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3), Sn2+(0.0004 mol·dm−3), As3+(0.053 mol·dm−3), Sb3+(0.004 mol·dm−3)]
Cu is a basic material used for electrical wire and rolled products. The grade of ore is deteriorating due to rising demand in developing countries, and the demand for the recycling of Cu has increased in Japan. As a result of this situation, the concentration of impurities included in crude Cu produced by pyrometallurgy has been increasing.1,2) Electrorefining of crude Cu obtained by pyrometallurgy produces Cu with a purity of above 99.99 mass%, which is used for electrical wire and rolled products. The anode for this electrorefining is usually crude Cu with a purity above 99 mass%.3–5) Passivation occurs when the Cu content in the anode is low, resulting in an increase in electrolytic voltage and no Cu dissolution. As a result, crude Cu with low Cu content of 80–95 mass%, i.e., the crude Cu with a high ratio of recycled raw material for smelting, cannot be directly applied for anode at this time. When the high purity Cu is produced from the low-grade Cu, the low-grade Cu is first powdered and then dissolved into an electrolytic solution, followed by electrowinning. For electrolysis, the electrorefining whose anode reaction is the dissolution of Cu requires approximately 300 kWh·t−1 of electric power consumption rate. However, because the insoluble anode such as Pb–Ag alloys is used (anode reaction is oxygen evolution) in electrowinning, the electrolytic voltage rises, resulting in a high electric power consumption rate for electrolysis of approximately 2,200 kWh·t−1.6) Even in smelting with low-grade crude Cu, the use of electrorefining is desirable from the standpoint of electric power costs.
Many studies have been published on the effect of impurities in the anode,7–15) the texture of the anode,16–19) electrolysis factor,20–22) and impurities ions7,23) and additives24–26) in electrolytic solution on the passivation behavior of anode in general electrorefining using crude Cu with the purity above 99 mass%. However, few studies on the passivation behavior of low-grade Cu anode with the purity of 80–95 mass% have been conducted. As a result, the authors electrolyzed using low-grade Cu anode with a purity of less than 80 mass% and found that the matrix phase of Cu anode dissolved in the form of dendrite and remained Cu–Ni–Sb–Sn–As compound formed the framework of slime at the stage of normal dissolution, and Cu, Ni, and Sn dissolved from the framework of Cu–Ni–Sb–Sn–As compound and the condensed phase of Sb was formed at the stage of passivation.27) It is critical to understand the relationship between anode passivation and electrolysis conditions in order to achieve electrorefining using the low-grade Cu anode. However, as far as the authors are aware, the effect of impurity ions and additives in electrolytic solution on the passivation of low-grade Cu anode has not been reported. Therefore, in this study, we performed the electrolysis using a low-grade Cu anode of purity less than 80 mass% and investigated the effect of impurity ions and additives in electrolytic solution on the passivation of Cu anode.
The low-grade Cu anode had a composition of 78.7 mass% Cu, 5.6 mass% Sb, 5.2 mass% Pb, 4.3 mass% Ni and 6.2 mass% other elements.
The impurities of Sb, Pb, Ag, and so on were confirmed to disperse in the grain boundary of Cu using backscattered electron images and energy dispersive X-ray spectroscopy of the cross-section of the anode.27) Although Ni was slightly detected at the grain boundary, it was assumed that it formed a solid solution in Cu.
The solution composition and electrodeposition conditions used in this study are shown in Table 1. A standard electrolytic solution was prepared by dissolving reagent-grade 0.708 mol·dm−3 CuSO4 (45 g·dm−3) and 2.04 mol·dm−3 H2SO4 (180 g·dm−3) in distilled and deionized water at room temperature. 100 mg·dm−3 of gelatin (Nippi Peptide, PA-10) with a mean molecular weight of 2000, 0.525 mmol·dm−3 of thiourea (40 mg·dm−3), and 1.13 mmol·dm−3 of chloride ions (40 mg·dm−3) in the form of HCl were added to the electrolytic solution after dissolving in distilled and deionized water at room temperature. Ni2+(0.596 mol·dm−3 (35 g·dm−3)), Bi3+(0.0005 mol·dm−3 (0.1 g·dm−3)), Sn2+(0.0004 mol·dm−3 (50 mg·dm−3)) ions were added in the form of sulfate, and Sb3+(0.004 mol·dm−3 (0.5 g·dm−3)), As3+(0.053 mol·dm−3 (4 g·dm−3)) and As5+(0.053 mol·dm−3 (4 g·dm−3)) ions were added in the forms of KSb(C4H2O6)·1.5H2O, As2O3 and H3AsO4, respectively, in standard solution as impurities. The concentrations of impurities above were set using data from a practical electrolytic solution of a Japanese Cu electrorefining.1,28,29) When the recycled low-grade Cu is used as an anode, the concentration of Ni2+ ions in solution is expected to rise due to increase in dissolution of Ni from the anode. As a result, even though the concentration of Ni2+ ions in a practical electrolytic solution is around approximately 0.34 mol·dm−3, the concentration in this study was set to be high. To investigate the effect of Cl− ions and thiourea on the passivation of Cu anode, the concentrations of Cl− and thiourea in solution were changed in some experiments.

99.99 mass% Cu sheet of 4 × 2 cm and a low-grade Cu sheet of 0.8 × 2.5 cm with back and sides sealed by epoxy resin were used as the cathode and anode, respectively. Electrodeposition was performed in an unagitated solution under a galvanostatic (200 A·m−2) condition at 60°C. Prior to electrodeposition, the anode was polished with emery papers (No. 600, 1500, and 2000) and buffed, thereafter the pickling for 180 s in 10 mass% H2SO4, electrolytic degreasing for 60 s, and the pickling for 180 s were performed as pretreatment. The cathode was polished with emery paper (No. 600). A saturated-KCl/Ag/AgCl reference electrode (0.199 V vs. NHE, 25°C) was used to measure the anode potentials during electrolysis, which were then plotted with reference to the NHE. Scanning electron microscopy (SEM) was used to examine the surface and cross-section of the anode after electrolysis and electron probe X-ray microanalysis (EPMA) and energy dispersive X-ray spectroscopy (EDX) were used to investigate the distribution of each element. They were analyzed using secondary electron images and reflection electron images during SEM observation. The slime layer exfoliated when the anode was cut to examine the cross-section because the slime on the anode was fragile after electrolysis. As a result, the resin embedding was conducted in such a way that the slime layer did not exfoliate during the cutting process. The Quetol-651 produced by Nisshin-EM corporation was used for resin embedding. The slime layer of the anode surface had numerous voids. Before resin curing, vacuum degassing was done to ensure that the voids in the slime layer were sufficiently embedded by the resin. The anode was cut by a diamond-cutter after resin curing, and the cross-section was polished with machine oil containing 0.3 µm alumina particles. Thereafter, the specimen was coated with carbon paste except for an observation area for SEM analysis, and the conductivity of the observation area was maintained with carbon shadowing. Figure 1 shows the schematic diagram of suspended electrolysis for anode observation. With time, the anode potential changed, and four stages emerged: 1) normal dissolution, 2) precursor of the passive film, 3) passivation and 4) disappearance of the passive film. The electrolysis was suspended at stage 3) passivation at which the anode potential was shifting to the oxygen evolution potential, and the surface and cross-section of the anode were observed.

Schematic diagram of suspended electrolysis for anode observation.
The diffusion coefficient of Cu2+ ions was measured using the rotating disk electrode (RDE) method under the condition with and without additives (gelatin, thiourea, Cl−) and impurities ions in solution shown in Table 1. With a wire electric discharge machine, 99.99 mass% Cu was cut into an 8 mm in diameter disk electrode (cathode). 99.99 mass% Cu of 3 × 5 cm was used as a counter electrode. The diffusion limiting current of the cathode reaction was measured from 0.34 V to −0.40 V using a potential-sweep method at 10 mV·s−1 under the rotation number of RDE of 50, 100, 150, 200, 250, and 300 rpm. Since the linear relationship is practical between the diffusion limiting current and ω1/2 (angular velocity, ω = 2πN, N denotes rotation number) according to Levich’s relational expression,30) the diffusion coefficient of Cu2+ ions was calculated from the inclination of the linear relationship.
The viscosity of the electrolytic solution was measured by the glass viscometers, Cannon-Fenske (SIBATA SCIENTIFIC TECHNOLOGY LTD).
To investigate the oxidative dissolution behavior of Sb, the anode current density was measured from 0.15 V to 1.0 V using a potential-sweep method at 1 mV·s−1 in an unagitated solution containing 2.56 mol·dm−3 H2SO4 and 0.053 mol·dm−3 As3+ or As5+ ions at 60°C. 99.99% mass% Sb sheet 2.2 cm in diameter and Cu sheet of 4 × 2 cm respectively with rear side sealed by epoxy resin were used as working and counter electrodes.
Figure 2 depicts the time dependence of potential of low-grade Cu anode during electrolysis at 200 A·m−2 in the solutions containing Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3), Sn2+(0.0004 mol·dm−3), As3+(0.053 mol·dm−3) or Sb3+(0.004 mol·dm−3) ions. The anode potential sharply shifted to a nobler direction at 18.3 hours of electrolysis in an impurity ions-free solution (CuSO4–H2SO4 solution containing gelatin, thiourea, and Cl− ions of standard concentration as additive). This is due to the passivation of the Cu anode. After a brief rise in anode potential, the anode potential shifted to a less noble direction, resulting in the return to normal electrolysis. Even at 18.5 hours after this, similar behavior was observed, but the passivation disappeared quickly. The time required for anode potential to rise to 1.0 V for the first time after the beginning of electrolysis was called passivation start time in this study. The electrolysis was repeated several times in an impurity ions-free solution, and the variation of passivation start time was approximately 0.5 hours in each experiment.

Time dependence of anode potential during electrolysis at 200 A·m−2 in the solutions containing various kinds of impurities. [Additive: Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3), Impurity: Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3), Sn2+(0.0004 mol·dm−3), As3+(0.053 mol·dm−3), Sb3+(0.004 mol·dm−3)]
The passivation start time was 11.6 hours in a solution containing 0.596 mol·dm−3 of Ni2+ ions, which was significantly shorter than the time to passivation in an impurity ions-free solution. In the solution in which As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions were previously added, the passivation occurred at 14.2 and 15.8 hours, respectively, and the passivation start time was shortened compared to that from impurity ions-free solution. In solution which Sn2+(0.0004 mol·dm−3) or As3+(0.053 mol·dm−3) ions were previously added, the passivation start times were 16.8 and 17.4 hours, respectively. When compared to the impurity ions-free solution, the time to passivation was slightly reduced. In contrast, the passivation start time in a solution containing 0.004 mol·dm−3 Sb3+ ions was nearly identical to that in a solution free from impurity ions. The effect of each impurity cannot be simply compared because the concentrations of added impurities ions in the solution were different. In the concentrations of impurities ions in this study, the passivation was promoted following the order: Ni2+(0.596 mol·dm−3) > As5+(0.053 mol·dm−3) > Bi3+(0.0005 mol·dm−3) > Sn2+(0.0004 mol·dm−3) > As3+(0.053 mol·dm−3) > Sb3+(0.004 mol·dm−3), that is, the harmful effect of Ni2+, As5+ and Bi3+ ions was large. The anode potential at the stage of passivation was constant (approximately 2.1 V), which is attributed to the anode potential reaching one for oxygen evolution.
Figure 3 depicts the time dependence of anode potential during electrolysis at 200 A·m−2 in the solutions containing various amounts of thiourea. Although Ni2+, As5+, Bi3+, Sn2+, As3+, and Sb3+ ions were not previously added to the solution, the standard concentration of gelatin and Cl− were contained in the solution. In the thiourea-free solution, the passivation start time was slightly prolonged. The passivation start time was slightly shortened when the concentration of thiourea was increased from the standard 0.525 mmol·dm−3 to 2.24 mmol·dm−3.

Time dependence of anode potential during electrolysis at 200 A·m−2 in the solutions containing various amounts of thiourea. (Gelatin: 100 mg·dm−3, Cl−: 1.13 mmol·dm−3, Impurity-free)
Figure 4 shows the relationship between the concentration of Cl− ions in solutions and the time to passivation of the anode. Despite the absence of Ni2+, As5+, Bi3+, Sn2+, As3+, and Sb3+ ions in the solution, the standard concentrations of gelatin and thiourea were present in the solution. The passivation start time was constant at the range of the concentration of Cl− ions of 0–1.13 mmol·dm−3 but it significantly shortened as the concentration of Cl− ions increased above 1.13 mmol·dm−3, indicating that the passivation was promoted with an addition of Cl− ions above 1.13 mmol·dm−3.
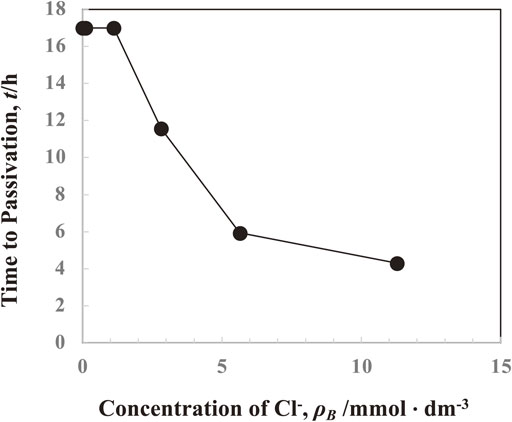
Effect of concentration of Cl− ions in solutions on the time to passivation of anode. (Gelatin: 100 mg·dm−3, Thiourea: 0.525 mmol·dm−3, Impurity-free)
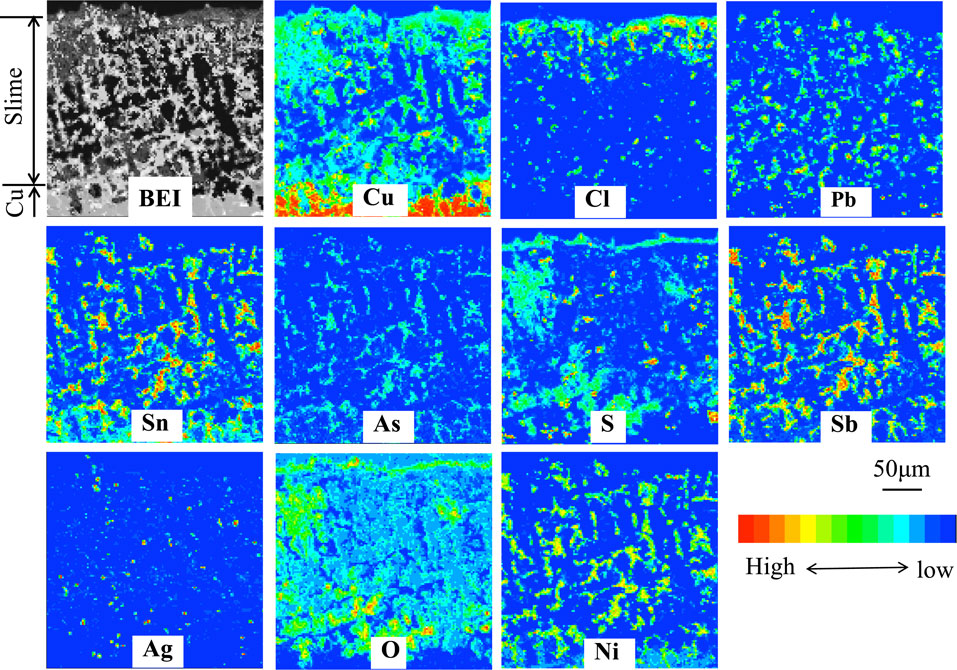
At the stage of passivation, the electrolysis was stopped while the potential was switched to one for oxygen evolution, and the surface and cross-section of the anode were examined. The texture and composition of anode slime that covered the anode surface at the stage of passivation were analyzed in a previous report using a low-grade Cu anode in an impurity ions-free solution.27) Figure 527) shows EPMA images of cross-sections of anode slime at the passivation stage in an impurity-free solution. At anode slime, Cu, Ni, Sb, Sn, As, Ag, Pb, S, O, and Cl were detected. Since Cu, Ni, Sb, Sn, As, Ag, and Pb were inherently present in the Cu substrate, the detected ones were residual or reprecipitated after Cu dissolution, showing that they were compounds difficult to dissolve. The overlapping of Pb and Cl, Ag and Cl, respectively, near the slime’s surface, was revealed by composition mapping images, indicating the formation of PbCl2 and AgCl. At the bottom of slime (i.e., vicinity of the surface of undissolved low-grade Cu substrate), Cu, S, and O concentrated, indicating that the passivation (i.e., the shift from Cu dissolution to oxygen evolution) is attributed to the formation of CuSO4 at the interface between the Cu substrate and slime. In EPMA analysis, although O was detected over the entire surface of slime, some resulted from the embedding resin-filled up in the voids in the slime layer. During electrolysis in a solution containing Ni2+ ions, the cross-section of anode slime at the stage of passivation was almost identical to that of Fig. 5.
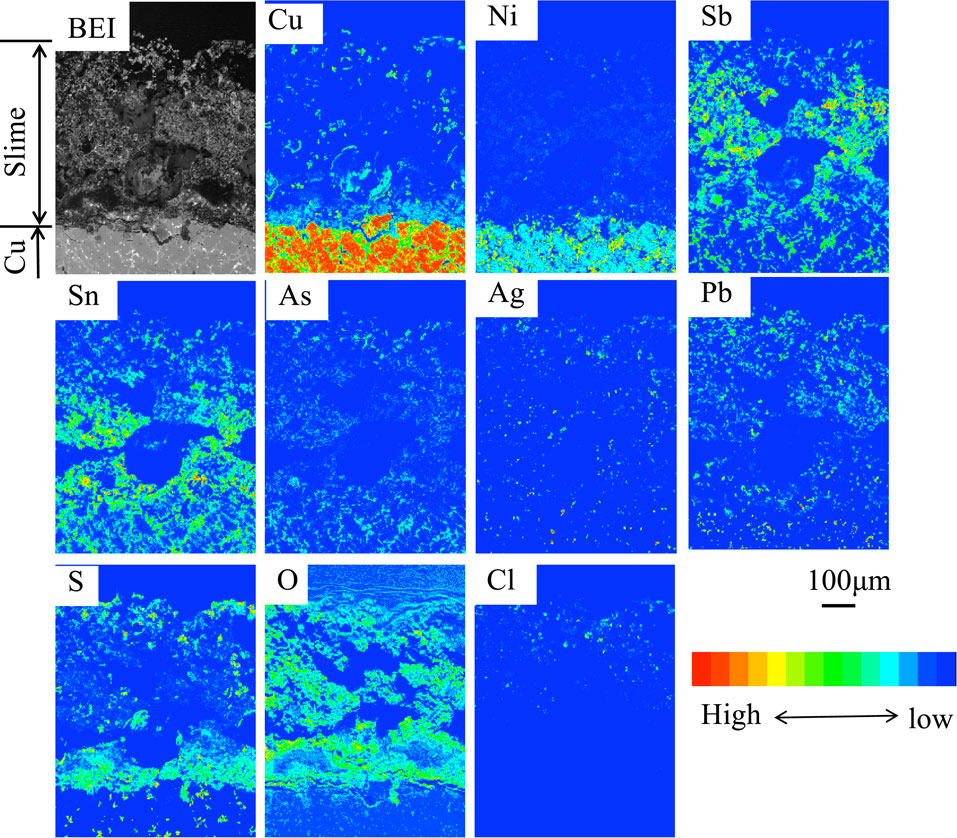
EPMA images of cross-section of anode slime at the stage of passivation in the impurity-free solution.27) [Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3)]
Figure 6 shows the EPMA images of a cross-section of anode slime at the stage of passivation during electrolysis in the solution containing 0.053 mol·dm−3 of As5+ ions. Cu, Ni, Sb, Sn, As, Ag, Pb, S, O, and Cl were detected at the anode slime in solution which As5+ ions were added, as with that from impurity ions-free solution (Fig. 5), but the concentration of As was higher over the entire slime. To learn more about anode slime, the slime was exfoliated from the Cu substrate, and a back surface mapping analysis was performed. Figure 7 depicts the outcome. As, Sb and O were significantly detected, in the lower right area of Fig. 7, indicating the formation of the As–Sb–O system compound at the inside of slime.

EPMA images of cross-section of anode slime at the stage of passivation in the solution containing 0.053 mol·dm−3 of As5+ ions. [Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3)]
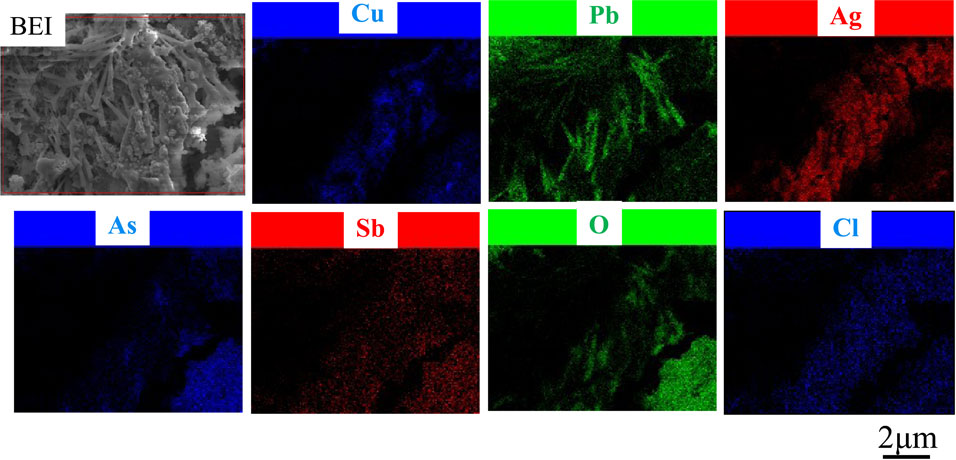
BEI image and EPMA mappings of back surface of anode slime at the stage of passivation in the solution containing 0.053 mol·dm−3 of As5+ ions. [Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3)]
Figures 8 and 9 show the EPMA images of the cross-section of the upper area of anode slime at the stage of passivation during electrolysis in the solution in which 0.0005 mol·dm−3 of Bi3+ ions were added. Red, blue, and green coloring were used to represent the three elements in slime. Although the areas where three elements coexist should be displayed in principle in white, they were displayed in a light yellow due to editing software. The presence of As, Bi, and O in the area of the white circle shown in Fig. 8, indicates the formation of the As–Bi–O system compound. In contrast, as shown in Fig. 9, there were areas where Bi, S, and O were strongly detected, indicating the formation of the Bi–S–O system compound. The compactness of slime is thought to increase because areas, where As–Bi–O or Bi–S–O system compound appears to be present, were observed at voids across the entire surface of the cross-section of slime.
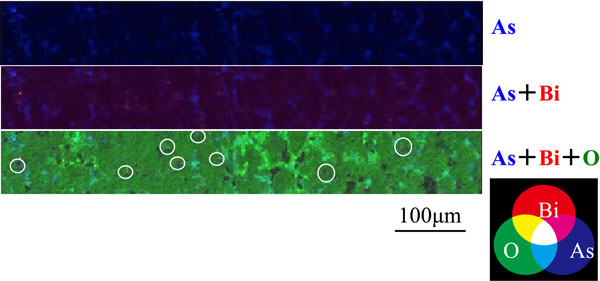
EPMA mappings of cross-section of upper area of anode slime at the stage of passivation in the solution containing 0.0005 mol·dm−3 of Bi3+ ions. [Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3)]

EPMA mappings of cross-section of upper area of anode slime at the stage of passivation in the solution containing 0.0005 mol·dm−3 of Bi3+ ions. [Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− (1.13 mmol·dm−3)]
The relationship between electrolysis duration and thickness of anode slime formed in solutions containing Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), or Bi3+(0.0005 mol·dm−3) ions are shown in Fig. 10. The apparent thickness of anode slime (average thickness including voids measured by observation of cross-section) was almost proportional to electrolysis duration, showing that Ni2+, As5+, and Bi3+ ions which were previously added rarely affected the apparent thickness of anode slime. Although the passivation start time was shortened in solutions containing Ni2+(0.596 mol dm−3), As5+(0.053 mol dm−3), or Bi3+(0.0005 mol dm−3) ions (Fig. 2), the apparent thickness of anode slime did not affect this phenomenon.

Relationship between electrolysis duration and thickness of anode slime in solutions with and without impurities. [Additive: Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− ions (1.13 mmol·dm−3), Impurity: Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3)]
Figure 11 shows EPMA images of cross-sections of anode slime at the passivation stage of electrolysis in a solution containing 5.64 mmol·dm−3 Cl− ions. After passivation, the electrolysis was immediately stopped (i.e., anode potential shifting to one for oxygen evolution). Cu and Cl overlapping at the upper area of the slime were observed in the solution containing 5.64 mmol·dm−3 Cl− ions, indicating the formation of Cu–Cl. Except for Cu, there was no significant overlap between Cl and other elements.
EPMA images of cross-section of anode slime at the stage of passivation in the solution containing 5.64 mmol·dm−3 of Cl− ions. [Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Impurity-free]
Cu anode passivation has been reported to be directly caused by the precipitate of CuSO4 at the interface between Cu substrate and slime in previous research.1,19,22) That is, the slime formed on the surface of Cu inhibits the diffusion of Cu2+ ions dissolved from the anode, and the concentration of Cu2+ ions exceeds the solubility of CuSO4 at the interface between Cu substrate and slime. As a result, CuSO4 precipitates on the Cu substrate, the passivation appears to occur, and the Cu dissolution reaction immediately shifts to oxygen evolution. The passivation was transient phenomenon, which is attributed to anode slime layer physically exfoliating due to oxygen evolution.
When the low-grade Cu, which contains a large amount of impurities such as Pb and Sb that are easily shifted to slime is used as anode like in this study, a larger amount of impurity slime is formed on the anode’s surface than the usual Cu anode at the stage of Cu dissolution. As a result, the diffusion of Cu2+ ions is inhibited, and the concentration of Cu2+ ions appears to increase. The type of impurities ions and concentration of additives in solution were found to affect the time from the start of electrolysis to passivation in this study. Therefore, the reason why the impurities ions and additives in the solution affect the passivation was discussed as follows:
The diffusion coefficient of Cu2+ ions and viscosity of the solution was investigated in solutions containing various impurities ions and additives. Figure 12 depicts the outcome. With the addition of As5+(0.053 mol·dm−3) and Bi3+(0.0005 mol·dm−3) ions, as well as additives (gelatin (100 mg·dm−3), thiourea (0.525 mmol·dm−3), Cl− ions (1.13 mmol·dm−3)), the diffusion coefficient of Cu2+ ions and viscosity of solution rarely changed. The viscosity of the solution increased with the addition of Ni2+ ions and the diffusion coefficient of Cu2+ ions decreased. Ni2+ ions were added by 0.596 mol·dm−3 in the form of NiSO4, that is, the feed ratio of SO42− ions also increased. The increase in the concentration of Ni2+ and SO42− ions in solution seems to increase the viscosity of the solution and decrease the diffusion coefficient of Cu2+ ions.
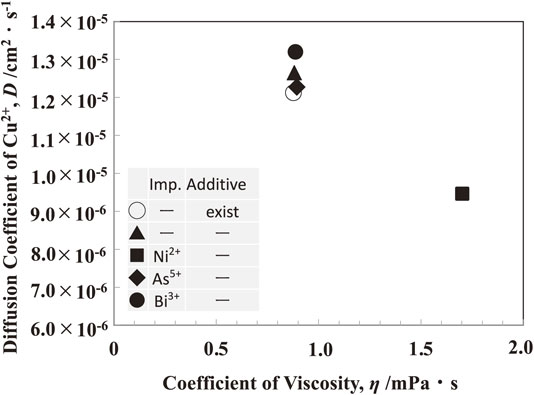
Effect of impurity and additive in solution on the diffusion coefficient of Cu2+ ions and coefficient viscosity of solution. [Additive: Gelatin (100 mg·dm−3), Thiourea (0.525 mmol·dm−3), Cl− ions (1.13 mmol·dm−3), Impurity: Ni2+(0.596 mol·dm−3), As5+(0.053 mol·dm−3), Bi3+(0.0005 mol·dm−3)]
The impurities ions and additives added to the solution were discussed from the viewpoint of their effect on the Cu passivation. As shown in Fig. 2, adding 0.596 mol·dm−3 of Ni2+ ions in the form of NiSO4 reduced the time to passivation to 11.6 hours. Ni2+ ions are reported to decrease the solubility of CuSO4,27) and decrease the diffusion coefficient of Cu2+ ions as shown in Fig. 12. This decrease in solubility of CuSO4 and diffusion coefficient of Cu2+ ions appears to promote the precipitate of CuSO4 in slime, resulting in a significant decrease in time to passivation. Since the concentration of Ni2+ ions dissolved from low-grade Cu anode during electrolysis for 20 hours is approximately 0.0006 mol·dm−3, the dissolution is negligible when compared to the concentration of Ni2+ ions which are previously added in the solution.
The insoluble SbAsO4 is known to be formed and promote the passivation when a certain amount of As5+ and Sb3+ ions are present in the electrolytic solution in the forms of H3AsO4 and SbO+, respectively.1,31) In this study, the low-grade Cu contains a significant amount (5.6 mass%) of Sb. During electrolysis, Sb, in addition to Cu, dissolves in solution as SbO+ ions. The time to passivation was shorter in a solution containing 0.053 mol·dm−3 of As5+ ions than that in a solution containing 0.053 mol·dm−3 of As3+ ions (Fig. 2). To discuss the difference in the effect of As5+ and As3+ ions in solution, the effect of As5+ and As3+ ions in solution on the oxidative dissolution behavior of Sb was investigated by linear sweep voltammetry (LSV). The result is shown in Fig. 13. In solution without As5+ and As3+ ions, the oxidation current of Sb (Sb + H2O → SbO+ + 2H+ + 3e−) became peak at 0.36 V, and it significantly decreased at potentials nobler than 0.36 V. The formation of passive Sb films is responsible for the decrease in oxidation current. Since three peaks of the oxidation current of Sb were respectively observed at 0.33, 0.37, and 0.4 V in a solution in which 0.053 mol·dm−3 of As3+ ions were added, the formation and destruction of passive films of Sb appear to repeat. At 0.37 V, the peak oxidation current of Sb was almost identical to that of the As3+ ions-free solution. In contrast, the peak oxidation current of Sb was observed at 0.27 V in a solution in which 0.053 mol·dm−3 of As5+ ions were added, and the peak value was significantly lower than in a solution containing As3+ ions. When the surface of Sb was analyzed by EDX after LSV in a solution containing As5+ ions, As, Sb, and O were detected over the entire surface, indicating the formation of As(V)–Sb(III)–O system compound (Fig. 14). This As(V)–Sb(III)–O system compound films seem to greatly suppress the dissolution of the metallic substrate.

LSV on Sb electrode in the solutions containing 0.053 mol·dm−3 of As3+ or As5+ ions. (H2SO4 2.56 mol·dm−3)
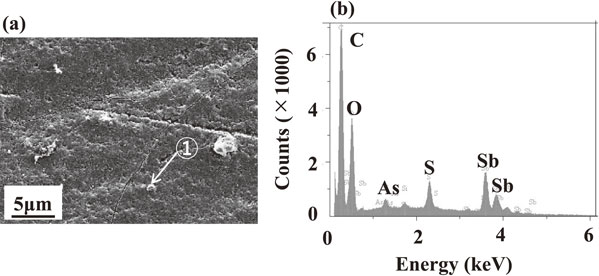
Surface observation and analysis of Sb electrode after LSV in the solution containing 0.053 mol·dm−3 As5+ ions, (a) SEM image and (b) EDX profile of ① in SEM image.
In the anode dissolution of low-grade Cu in this study, As, Sb and O were detected in anode slime formed by electrolysis in a solution containing As5+ ions (Fig. 7), SbAsO4 was anticipated to be formed in slime. Although SbAsO4 was not the main constituent of the anode slime, the formation of the network of SbAsO4 inside the slime layer may have suppressed the diffusion of Cu2+ ions. That is, although the apparent thickness of slime is not thick, the diffusion of Cu2+ ions in slime is significantly suppressed, resulting in a decrease in passivation start time.
Bi2O3 and Bi2(SO4)3 are known to be formed in slime in an electrolytic solution containing Bi3+ ions,7) whereas BiAsO4 is reported to be formed on Cu anode and promote the passivation in a solution containing a certain amount of As5+ and Bi3+ ions.1,31) The areas where Bi, S, and O (Fig. 9) and As, Bi, and O (Fig. 8) were respectively detected in slime increased when the electrolysis was performed in a solution in which 0.0005 mol·dm−3 of Bi3+ ions were added. Because the presence of 0.0005 mol·dm−3 of Bi3+ ions in solution had little effect on the apparent thickness of slime (Fig. 10), the compactness of slime may increase due to formations of Bi2O3, Bi2(SO4)3, and BiAsO4, promoting passivation.
Cu and Cl overlapping were observed at the surface of anode slime when the concentration of Cl− ions in solution was increased from 1.13 mmol·dm−3 (standard) to 5.64 mmol·dm−3 (Fig. 11). Figure 15 depicts the potential-pCl diagram of the Cu–Cl–H2O system. The concentration and activity coefficient of each ion was assumed to be 0.7 mol·dm−3 and 1, respectively, when creating this potential-pCl diagram. Paurbaix32) and Sillen33,34) were consulted for the thermodynamic data used. The dissolved Cu precipitates in form of CuCl according to the concentration of Cl− ions in the solution, as shown in the potential-pCl diagram of the Cu–Cl–H2O system shown in Fig. 15. At the concentration of Cl− ions of 5.64 mmol·dm−3, assuming that the activity coefficient is 1, pCl− in solution is approximately 2.25. However, Cl− ions are known to specifically adsorb on the surface of metal,35,36) pCl− at the surface of Cu substrate at the initial stage of electrolysis is expected to be lower than 2.25. CuCl is thought to be formed at the initial stage of electrolysis in a solution containing Cl− ions of high concentration (approximately 5.64 mmol·dm−3). The passivation start time was short in electrolysis in a solution containing 5.64 mmol·dm−3 of Cl− ions. The precipitates of the chloride system formed at the surface of anode slime appear to compact compared to internal texture and have a strong suppression effect on the diffusion of Cu2+ ions. If the suppression effect of precipitates of chloride system on the diffusion of Cu2+ ions is small, the precipitates of chloride system formed at the initial stage of electrolysis may increase the compactness of anode slime formed later and cause the passivation easily.
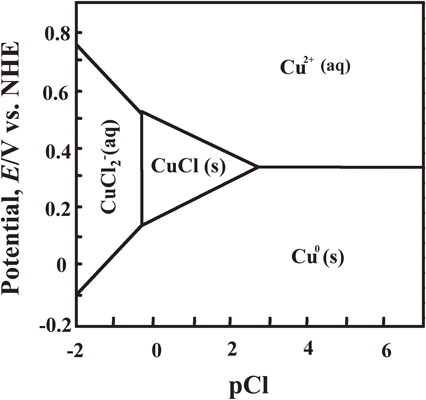
Potential-pCl diagram of Cu–Cl–H2O system at 25°C. (Activity of each ion species: 0.7, pH 0).
Cu+ ions increase the slime due to the formation of Cu powder by the disproportionation reaction (2Cu+ → Cu + Cu2+), resulting in promotion of passivation.1,25) Thiourea is said to suppress the passivation when added at a concentration of approximately 0.0026 mmol·dm−3.25) This can be explained by the fact that thiourea decreases Cu+ ions that cause the passivation due to the formation of a complex with Cu+ ions. In contrast, increasing the concentration of thiourea is reported to promote passivation.25) Thiourea decomposes by hydrolysis in electrolytic solution or oxidation on an anode, and this decomposition product is reported to promote the formation of passive films. Passivation was promoted in this study because the added concentration of thiourea in the solution was high (above 0.525 mmol·dm−3) (Fig. 2). The decomposition product of thiourea formed during electrolysis seems to promote passivation.
Finally, the difference in the effect of impurities ions and additives in solution on the passivation between low-grade Cu with the purity of 78.7 mass% in this study and usual Cu with purity above 99 mass% was discussed below. When the impurities ions that promote the formation of slime are present in solution, the time to passivation is reported to be significantly reduced in normal Cu with a purity of about 99 mass%.7) For example, As3+, Bi3+, and Sb3+ ions increase the amount of formation of slime by increasing the concentration in solution and promote the passivation, while Ni2+ and Sn2+ ions rarely form the slime and do not affect the passivation at the concentration level of 2000 ppm.7) In low-grade Cu in this study, Bi3+ ions added by 0.0005 mol·dm−3 in solution promoted the passivation, whereas Sb3+ ions which were added by 0.004 mol·dm−3 in solution had little effect and effect of addition of 0.053 mol·dm−3 of As3+ was also small. Because the amount of formation of slime formation in low-grade Cu is inherently large, impurities ions that alter slime characteristics such as kinds of compound and compactness to obstruct the diffusion of Cu2+ ions are thought to promote the passivation. Since the As–Sb–O and As–Bi–O system compounds promoted the passivation, they appear to increase slime compactness. In this study, the effect of the addition of Sb3+ ions on the passivation was rarely observed, which is attributed to Sb3+ ions being dissolved from low-grade Cu anode during electrolysis. In contrast, with respect to the effect of Cl− ions in solution on the passivation of the usual Cu anode, there are directly-opposed reports that Cl− ions in solution promote26) and suppress25) the passivation. Cu+ ions cause passivation to decrease due to the formation of soluble complex ions of CuCl2−, which explains why Cl− ions suppress passivation.25) The formation of CuCl is said to be the reason why Cl− ions promote passivation,26) but the degree of promotion is small in normal Cu. In this study, however, Cl− ions significantly promoted the passivation of low-grade Cu, which differed significantly from the passivation behavior of ordinary Cu. Because the amount of slime formation is inherently high in low-grade Cu, the synergistic effect of slime and CuCl appears to promote passivation significantly.
Cu electrolysis was performed employing a low-grade copper anode with a purity of 78.7 mass%, and the effect of impurities ions and additives in solution on the passivation behavior of the anode was investigated. In a solution containing 0.596 mol·dm−3 of Ni2+ ions as an impurity, the time when anode passivation first occurs shortened significantly, and in a solution containing As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions, it shortened somewhat. Sn2+(0.0004 mol·dm−3) and As3+(0.053 mol·dm−3) ions reduced the time to passivation slightly, but Sb3+(0.004 mol·dm−3) ions rarely did. In the case of concentration of impurity in this study, the degree of harmful effect of impurity ions on the passivation follows the order: Ni2+(0.596 mol·dm−3) > As5+(0.053 mol·dm−3) > Bi3+(0.0005 mol·dm−3) > Sn2+(0.0004 mol·dm−3) > As3+(0.053 mol·dm−3) > Sb3+(0.004 mol·dm−3). The viscosity coefficient of the solution increased when the 0.596 mol·dm−3 of Ni2+ ions were added while the diffusion coefficient of Cu2+ ions decreased. The compound of the As–Sb–O or As–Bi–O system was formed in anode slime when the As5+(0.053 mol·dm−3) or Bi3+(0.0005 mol·dm−3) ions were added to the solution, which seemed to increase the compactness of slime. The time to passivation was slightly longer in thiourea-free solution but reduced when the concentration of thiourea was increased from 0.525 to 2.24 mmol·dm−3. In the solutions containing 0 to 1.13 mmol·dm−3 of Cl− ions, the time to passivation was constant, but significantly shortened with increasing the concentration of Cl− ions at the region above 1.13 mmol·dm−3. Cl− ions formed Cu–Cl at the upper area of anode slime, as a result, increased the compactness of slime and promoted passivation.
This work was supported by JSPS KAKENHI Grant Number JP 21K18829.