2023 年 64 巻 1 号 p. 303-305
2023 年 64 巻 1 号 p. 303-305
The electrodeposition of Si metal in molten CaCl2 containing various types of calcium silicate has been studied at 1373∼1673 K. It was shown that the influence of the molar ratio of CaO to SiO2 on Si electrodeposition was not remarkable in contrast with our previous results that the molar ratio of CaO to TiO2 strongly affected the Ti metal deposition in the similar system. Silicon metal was obtained on MoSi2 cathode in the melt by potentio-static electrolysis. The cathodic current efficiency was about 30% regardless of the added types of calcium silicate. The current efficiency tended to be better as the electrolysis potential became negative, but the electrolysis below the border potential lead to lower current efficiency due to Ca co-deposition.
Enough metallic Si to evaluate the current efficiency from its weight was obtained by potentio-static electrolysis in molten CaCl2 with Ca2SiO4 at 1673 K. It was shown that the current efficiency of Si metal deposition depended on electrolysis potential, though the further study to clarify the optimum condition for better electrolysis is necessary.
Metallic Si is produced by carbon reduction of SiO2 in an arc furnace above 2000 K. Since the energy consumption and CO2 exhaustion in the process are very large, an energy-efficient and environment-friendly process is desired. Si electrolysis in molten CaCl2 has been studied by several groups.1–4) The authors have also researched the Si electrodeposition in molten CaCl2 containing CaSiO3 above 1273 K to obtain metallic silicon in liquid; CaSiO3 dissolved in molten CaCl2 at 1573 K, and the cathodic reaction of Si occurred.5) A thick MoSi2 layer was formed on Mo cathode by potentio-static electrolysis and metallic Si was detected in the electrodeposit. These results suggested that the cathodic reduction to metallic Si could be possible in the system. However, the suitable condition of Si metal deposition had not been studied in detail.
In this study, various types of calcium silicate were used as solute, and the dependence of cathodic reaction on the molar ratio of CaO to SiO2 (RCaO/SiO2, hereinafter) of calcium silicate was investigated based on our previous study in molten CaCl2 with calcium titanate.6,7) The electrochemical behavior of calcium silicate was analyzed by cyclic voltammetry, and potentio-static electrolysis was performed. MoSi2 electrode was used as the cathode to prevent further MoSi2 formation, and the dependence of the cathodic current efficiency on the electrolysis potential was discussed.
Commercial CaSiO3 (Wako Pure Chemical, CaO: 40.0∼48.0, SiO2: 51.0∼57.0%) and calcium silicates of various RCaO/SiO2 values synthesized from CaO (Kishida Chemical, >98%) and SiO2 (Kojundo Chemical Laboratory, >99.9%) were used. It was confirmed by that the synthesized calcium silicate consisted of Ca2SiO4, Ca3Si2O7 and CaO. Their compositions changed with RCaO/SiO2, which were consistent with the phase diagram of the CaO–SiO2 binary system.8) In this paper, molten CaCl2 containing the calcium silicate of RCaO/SiO2 = 1.5 is simply written as “the bath of RCaO/SiO2 = 1.5”, for example, and the concentration of calcium silicate was 5.0 mol% based on the SiO2 concentration.
The apparatus for electrochemical measurement and electrolysis was almost the same as in our former studies.5) Calcium chloride (Kishida Chemical, >95%) containing the calcium silicate was put into a Mo crucible (3N5, Sanwa Metal), and vacuum-dried at 373 K for a day. The salt in a Mo crucible was set in an air-tight furnace tube in an electric furnace (Motoyama, MS-2821). The system was heated, and the salt was held at 1373∼1673 K under a high-purity Ar atmosphere.
A MoSi2 rod (Kanthal Super 1700, ϕ3.0 mm) of which active surface area limited with an Al2O3 sheath was used as the working electrode for cyclic voltammetry. A MoSi2 electrode with a BN receiver (TEP, pure grade) as shown in Fig. 1 was also used at 1673 K to collect the electrodeposit efficiently for potentio-static electrolysis. A graphite rod (Nippon Techno-Carbon, MF307, ϕ5.0 mm) was used as a counter electrode. A Mo wire was used as a quasi-reference electrode, and its potential was calibrated versus the Mo dissolution potential measured by cyclic voltammetry.
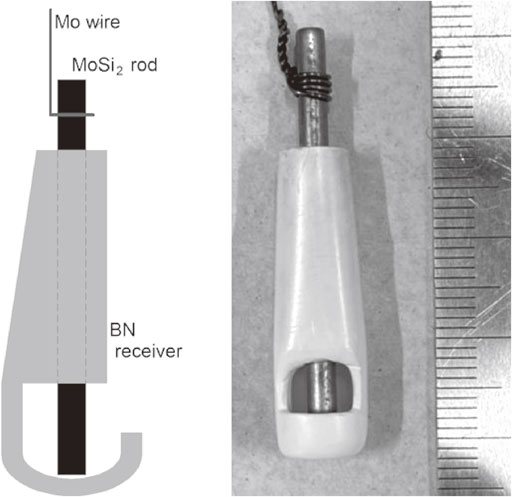
MoSi2 cathode with BN receiver.
The electrochemical behavior of Si was investigated by cyclic voltammetry, and then potentio-static electrolysis was performed. The electrodeposit was analyzed by XRD (Rigaku, RINT-2550V) and SEM (JEOL, JCM-6000) - EDX (JEOL, EX-5441).
Cyclic voltammograms in the baths of RCaO/SiO2 = 1.0, 1.5 and 2.0 at 1373 K are shown in Fig. 2. In the bath of RCaO/SiO2 = 1.0, a reduction current peak was observed at −1.3 V. A current bulge was seen around −1.2 in the bath of RCaO/SiO2 = 1.5, and around −1.4 V in the bath of RCaO/SiO2 = 2.0. Considering the results of potentio-static electrolysis mentioned after and the report that one-step reduction of Si4+ + 4e− → Si occurred in the LiF–NF–KF–SiO2 system,9) it is thought that silicate ion was reduced to Si metal by one step under the condition in this study. The reduction step seemed not to depend on the RCaO/SiO2 value, but the reduction potential was influenced.

Typical cyclic voltammograms at 1373 K.
Clear current peaks or bulges could not be observed in the cyclic voltammogram above 1573 K. It is considered that stronger convection in the bath at a higher temperature affected the cyclic voltammogram.
Potentio-static electrolysis was performed based on the results of the cyclic voltammogram, and a deposit piled up on the MoSi2 cathode in all the baths of RCaO/SiO2 = 1.0, 1.5 and 2.0 at 1573 K. Metallic Si particle was contained in the deposit, and the bath components were included. The XRD pattern of the typical deposit after rinsing is shown in Fig. 3. The deposit mainly consisted of metallic Si, and silicon carbide was not detected in it. The RCaO/SiO2 value influenced the deposit; metallic Si seemed to be obtained more easily in the baths of RCaO/SiO2 = 1.5 and 2.0 than in the bath of RCaO/SiO2 = 1.0. The electrolysis potential also affects the deposition, and CaSi2 was detected in the deposit according to the condition.
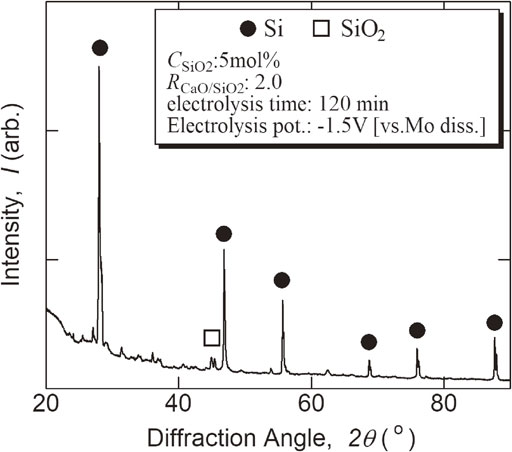
XRD pattern of electrodeposit obtained by potentio-static electrolysis at 1573 K.
A thick layer of Si metal was formed by potentio-static electrolysis at 1673 K as shown in Fig. 4. Oxygen was slightly detected due to the surface oxidation during the sample preparation, while Ca was not contained in the deposit. The graphite anode was consumed by electrolysis. Considering the temperature and the contents of cathode deposit, it is thought that the reaction: C + O2− → CO + 2e− occurred at the anode.

SEM image and elemental distributions of cross section of MoSi2 electrode with electrodeposit obtained at 1673 K.
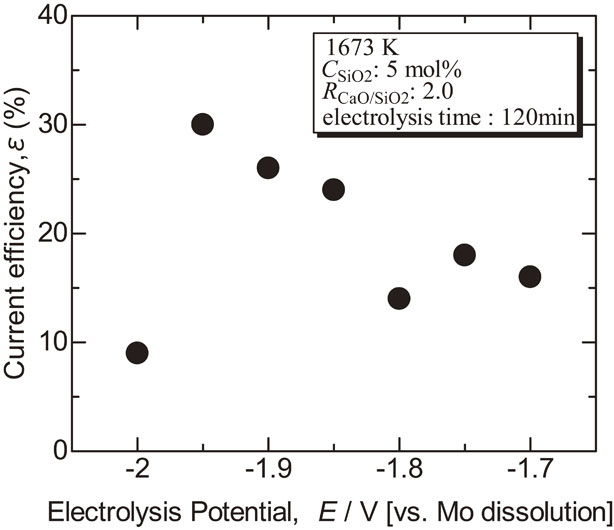
Figure 5 shows the current efficiency calculated from the weight of electrodeposit and the electric quantity during electrolysis assuming the reaction: Si4+ + 4e− → Si. The current efficiency tended to better as the electrolysis potential became negative, but it dropped sharply at −2.0 V. CaSi2 was detected from the deposit by the electrolysis at −2.0 V, so co-deposition of Ca might hinder metallic Si deposition.
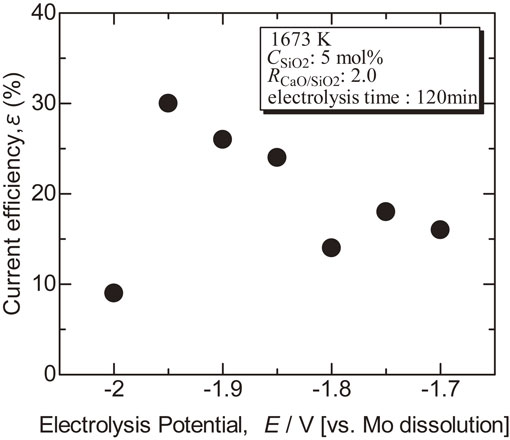
Dependence of current efficiency for Si electrodeposition on electrolysis potential in molten CaCl2 with Ca2SiO4 at 1673 K.
It was clearly indicated that metallic Si could be obtained by electrolysis in molten CaCl2 containing calcium silicate. However, the current efficiency was still limited to around 30% at most. Since it was shown that RCaO/SiO2 and electrolysis potential strongly affected the Si deposition as mentioned above, the detailed study on their influence should be necessary for better electrolysis. Metallic Si was deposited in solid under the condition in this study, so the detachment by strong convection in the bath might cause the low current efficiency. The electrolysis above the melting point of Si should be also attempted.
Metallic Si was electrodeposited by one-step reduction; the electrolysis potential and temperature strongly influenced the Si deposition, whereas the dependence on RCaO/SiO2 value was not remarkable as compared with our former results on Ti deposition in molten CaCl2 containing calcium titanate. A thick metallic Si layer was formed on MoSi2 cathode by the potentio-static electrolysis at 1673 K, and the current efficiency was about 30% under the suitable condition. A detailed study to realize more efficient electrolysis should be necessary, and also the research to obtain metallic Si in liquid should be desirable.
The study was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant-in-Aid for Scientific Research (B), #18H01763).