2023 年 64 巻 1 号 p. 25-30
2023 年 64 巻 1 号 p. 25-30
Metal additive manufacturing is a powerful tool for providing the desired functional performance of hard tissue biomaterials through a three-dimensional structural design. It is essential to use the interactions between living organisms and materials for functional hard tissue reconstruction. In particular, anisotropic high-performance materials that imitate bone tissue properties are required for regaining bone functionality based on collagen/apatite microstructure. This review article describes the current development of controlling hard tissue compatibility by additive manufacturing of titanium alloys, including our recent findings on the bone medical devices for guiding the anisotropic bone microstructure.
This Paper was Originally Published in Japanese in J. JILM 72 (2022) 339–343. The title was changed due to the addition of “Review—”.
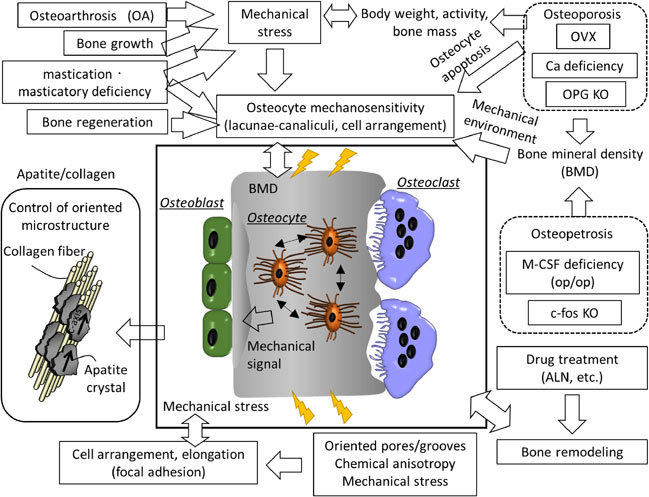
Fig. 4 Various factors that control anisotropic bone microstructure. Bone orientation is tightly controlled by various kinds of genes, diseases, cell adhesion mechanisms, and cell-cell communication molecules. These are the most important factors to consider in developing hard tissue compatible bone medical materials.
Among light metal materials, titanium and its alloys show high mechanical reliability and excellent hard tissue compatibility and are widely used in dentistry and orthopedics as medical metal materials. Metal materials in contact with bone tissue must exhibit hard tissue compatibility. It is necessary to ensure that they can organically interact with proteins and cells after implantation in the living body, as well as to facilitate functional recovery and eliminate harmful effects such as cytotoxicity and bacterial infections. To achieve this aim, it is essential to understand the mechanism of functional expression in living tissues and organs and develop materials to induce them. In particular, the key to recovering lost bone function is the induction of the expression of functionality required in three dimensions, based on the anisotropic structure of the original bone tissue. Metal additive manufacturing enables structural control and also atomic arrangement. It is widely regarded as a powerful next-generation technology for realizing anisotropic high-performance materials that imitate bone function. This paper describes the current development of controlling hard tissue compatibility by three-dimensional modeling of titanium alloys. In addition, we outline the latest findings of bone medical device research for guiding anisotropic bone microstructure.
The powder bed fusion method, a type of metal additive manufacturing, creates a three-dimensional structure by melting/solidifying the metal powder based on the 3D CAD data of the final shape. Therefore, it is possible to realize complicated shape control, internal porosity, and high-precision control of the surface shape, all of which are difficult to achieve with conventional casting and cutting methods. Using the powder bed fusion method and controlling the internal and external complex shapes, it is possible to control the crystal texture based on optimizing the heat source scanning strategy. It is expected to be a next-generation technology that directly leads to more functional bone medical devices.1,2) Control of crystallographic texture enables physical property control based on the orientation dependence of mechanical properties, such as Young’s modulus, leading to the creation of implants that can suppress stress shielding.3) By taking advantage of these features, we expect to realize the design of bone medical devices with high biocompatibility from both mechanical and biological aspects.
Porous structures have been proposed to lower Young’s modulus and improve the hard tissue compatibility of structures generated using metal additive manufacturing (Table 1).4–15) The porosity and pore size in structure induce changes in body fluid inflow, cell invasion, proliferation, and differentiation ability. The control of complex internal shapes by this method is extremely effective in inducing the direct coupling of the device with bone. Due to forming a strong passivation film, titanium and its alloys exhibit excellent corrosion resistance and hard tissue compatibility. Furthermore, they have osseointegration properties (direct coupling with bone at the light microscope level). However, the shape of the material surface significantly affects the manifestation of these characteristics. It has been reported that control of the structural surface shape by scanning electron beam or laser beam induced osteoblast proliferation,16,17) collagen matrix secretion,18) and stem cell differentiation,19–21) all of which depend on the surface roughness. Furthermore, in recent years, it has been reported that shaping direction and process parameters also control the proliferation and gene expression of stem cells.22–24) The functional control of cells starting from such material surface structure-biological interface interactions is thought to be due to biological responses. The cellular responses, including cell adhesion through protein adsorption, transcription factor expression in the cell nucleus, have been proposed as a cell guiding cue by structural control of metal materials. For functional bone reconstruction, it is essential to effectively use these interactions between living organisms and materials and connect the bone-device interface with a functionally fused bone. Bone exerts the necessary functionality in three dimensions by hierarchically constructing anisotropic tissue. Therefore, the formation of anisotropic tissue is indispensable for the soundness of the bone, which needs to start from the interactions between the bone medical device and living tissue. The authors have developed bone medical devices that can induce bone functionalization based on the anisotropic microstructure peculiar to living bone by using metal additive manufacturing technology.4,25,26)

Bone tissue is mainly composed of collagen/apatite crystals. The c-axis orientation of anisotropic apatite crystals along the direction of collagen fibers changes extensively, reflecting the stress distribution in a living body.27) In particular, the collagen/apatite crystal c-axis shows an anisotropic arrangement for the maximum principal stress vector direction and thus shows high strength in the orientation direction.28) Living bone exhibits high-strength characteristics in the required direction due to such crystal aggregates. The crystallographic anisotropy of the bone varies greatly depending on the anatomical site. Figure 1 shows the c-axis orientation of apatite crystals in three orthogonal directions in the cortical bones of the ulna, skull, mandible, and lumbar spine. The ulna and lumbar vertebrae show preferential orientation along the long bone and craniocaudal axes, respectively, reflecting the in vivo uniaxial stress load. In contrast, the mandible shows a three-dimensional orientation distribution that reflects the masticatory load, suggesting a close relationship between in vivo stress distribution and bone orientation. However, it is difficult to spontaneously reconstruct bone orientation in diseased and regenerated bones that have lost their appropriate anisotropy, even if bone density is substantially recovered.29–32) To regain bone function, developing a medical device that artificially induces the repair of lost bone orientation is indispensable. The use of artificial materials to express anisotropy function in the interface between living tissues is key to achieving this aim.

Collagen/apatite crystal orientation in typical cortical bone. The c-axis self-assembles almost in parallel according to the anatomical site. Long bones and vertebral bones show a uniaxial orientation, while skulls show a two-dimensional orientation, and mandibles show a complex orientation depending on the site. It exhibits anisotropic bone orientation according to the in vivo stress distribution and optimum mechanical properties. Modified based on Ref. 27).
To establish the anisotropic bone reconstruction, it is important to control the unidirectional arrangement of osteoblasts, the cells responsible for bone regeneration. The controlled arrangement of osteoblasts can be artificially achieved using the surrounding environment by altering parameters such as material surface shape,33–35) anisotropy of strain/stress load,36) and chemical anisotropy due to collagen fiber orientation.37,38) In each case, the bone matrix constructed by the osteoblasts is parallel to the cell direction; the integrin-mediated adhesion mechanism between the cell and substrate is considered a trigger for anisotropic bone matrix formation. Another determinant for bone matrix orientation is the anisotropy of the collagen secretion pathway by intracellular vesicle transport. The sensing process of material surface shape, the subsequent cell alignment, and oriented bone matrix organization is precisely controlled by the molecular mechanism of the material-biological interface reactions. Integrins activate the maturation of the actin cytoskeleton and focal adhesion by signaling from outside the cell to the inside, regulating cell functions. Micrometer-scale surface topography based on plastic deformation of metal single crystals and nanometer-accurate microfabrication by lithography can also achieve unidirectional cell alignment and further bone matrix orientation by inducing a unique molecular cascade characteristic for organelle functions.39) In contrast, metal additive manufacturing, which can help control surface structures on the sub-millimeter scale, enables fluid inflow into the guidance of cellular components to the device surface.40) Figure 2 shows the arrangement of mesenchymal stem cells along a unidirectional pattern formed by a laser beam with a groove width of 250 µm. Focal adhesions recognize the ripple structure due and induce cell elongation. The arranged mesenchymal stem cells differentiate into mature osteoblasts via the activation of transcription factors and serve as the starting point for forming an oriented bone matrix. This demonstrates that surface structural control is important for cell anisotropy and the resultant regeneration of oriented bone matrix microstructure. It enables functional bone reconstruction from the initial regeneration stage even without loading.

Arrangement of mesenchymal stem cells along a unidirectional orientation grooves formed by L-PBF (visualization of actin, one of the cytoskeletal proteins, and focal adhesions).
Once bone formation around the device is achieved, the continuous transmission of the maximum principal stress vector to the bone is essential for bone health. A dental implant designed for anisotropic bone microstructure was proposed, approved, and marketed in 2017.41) Furthermore, designing an oriented groove structure on the surface of an artificial hip joint stem that enables the guidance of oriented bone microstructure into the groove received regulatory approval in 2018.42) A device for spinal fusion that can induce orientation in the bone was approved by regulatory affairs in April 2021 and launched in July 2021 (Fig. 3).43) The developed vertebral fusion cage device has been produced using metal additive manufacturing and features the design of a special microstructure (honeycomb tree structure/HTS). This characteristic structure enables the oriented bone ingrowth with preferential alignment of bone matrix microstructure. It is possible to obtain excellent bone fusion without a large amount of autologous bone grafting. The finely-oriented groove structure on the surface of the device brings about the unidirectional extension and arrangement of osteoblasts. It reconstructs oriented bone tissue from the early stages of bone regeneration.44) This results from the anisotropic structure of the artificial material surface, which induces anisotropy in the cytoskeleton and adhesion, leading to the formation of an anisotropic bone matrix based on the production of oriented collagen and epitaxial growth apatite crystals in the presence of Osteocalcin protein.45) Bone matrix orientation starting from such a cell arrangement is controlled by a molecular mechanism mediated by the activation of focal adhesion by integrins, as described above. It is also possible to control bone matrix orientation genetically.46) The presence of genes, cell adhesion mechanisms, and cell-cell communication substances that cause such orientation are among the most important factors in developing next-generation medical devices with hard tissue compatibility (Fig. 4).

Development of spinal fusion devices focused on anisotropic bone microstructure. (a) A new porous structure (HTS/honeycomb tree structure) that results in early oriented bone formation on device implantation, (b) collagen fibers oriented in the cranio-caudal direction are formed, and (c) osteocytes are preferentially arranged.

Various factors that control anisotropic bone microstructure. Bone orientation is tightly controlled by various kinds of genes, diseases, cell adhesion mechanisms, and cell-cell communication molecules. These are the most important factors to consider in developing hard tissue compatible bone medical materials.
In recent years, developing three-dimensional organs and organoid research using living cells as raw materials using bioprinting technology has advanced and is expected to be applied in regenerative medicine. The authors created an anisotropic mini-bone organ similar to the bone by controlling single-cell drawing and molecular arrangement of protein using inkjet bioprinting.47) Inside the mini-bone organ, stress-sensitive osteocytes constructed a network structure from which cell processes extended three-dimensionally. The stress applied to the bone is sensed by the integrin molecules on the surface of the osteocyte as a fluid flow in lacuno-canalicular system inside the bone matrix and regulates bone remodeling by transmitting biochemical signals to the osteoblasts. Osteocytes inside mini-bone organs were shown to modify the process structure in response to fluid stimuli and, by communicating with osteoblasts, served as the origin of biological signals for oriented bone matrix microstructure. Cell control that makes full use of such bioprinting technology will lead to the creation of medical devices for inducing bone functions and is expected to elucidate the mechanism of anisotropy formation in the living body. It is expected to find a wide range of applications within fields such as drug discovery. In addition, the creation of oriented bone-like structures by the three-dimensional drawing of biopolymer materials, such as cells and proteins, is expected to be realized in the form of a bone-filling material that exhibits a high degree of hard tissue compatibility with good mechanical strength. It is expected to combine such biochemical approaches with additive manufacturing of titanium and its alloys. There are high expectations that this will be effective for the realization of early-oriented regenerated bone and can also be used as a drug efficacy evaluation platform.
Focusing on the collagen/apatite-oriented structure, indispensable for the healthy exertion of the mechanical and biological functions of bone, we have introduced research on bone medical devices for bone health. Next-generation bone devices guiding anisotropic bone matrix microstructure from the initial stage of implantation can be established by controlling the material properties for cellular arrangement. Metal additive manufacturing is extremely useful as a technology for realizing the functionalization of such materials. In addition to controlling complex shapes inside and outside, it is possible to freely exert functions by controlling materials such as crystal texture and atomic arrangement. In particular, control of the surface shape by additive manufacturing effectively controls cell anisotropy, which determines the oriented bone mictostructure and enables the expression of bone anisotropic functions. It is based on interactions between the surface topography and cells via focal adhesions. Bone orientation control by implants that make full use of metal additive manufacturing will also provide bone medical devices customized for each bone site and patient through integration with digital transformation (DX) technology by anisotropy of bone mechanical functions such as Young’s modulus. The creation of hard tissue-compatible bone medical devices via additive manufacturing is expected to lead to the proper functionalization of bone tissue as a structural and functional material, based on the concept of biological bone anisotropy.
This work was supported by a Grant-in-Aid for Scientific Research (JP18H05254) from the Japan Society for the Promotion of Science (JSPS) and CREST-Nanomechanics: Elucidation of macroscale mechanical properties based on understanding nanoscale dynamics of innovative mechanical materials (Grant Number: JPMJCR2194) from the Japan Science and Technology Agency (JST).