2023 年 64 巻 1 号 p. 61-70
2023 年 64 巻 1 号 p. 61-70
To promote the recycling of Ti scrap, it is essential to develop new technologies that can efficiently remove oxygen impurities from the Ti scrap. However, the direct removal of oxygen dissolved in solid Ti is extremely difficult, and currently, there are no effective deoxidation methods that can be used industrially. In this study, we experimentally verified a new deoxidation technique for Ti using the vapor of rare earth metals with high vapor pressures, such as Sm, Eu, Tm, and Yb. It was confirmed that Eu did not decrease the oxygen concentration in the Ti samples below 1000 mass ppm. On the other hand, it was shown that the vapor of Sm, Tm, and Yb decreased the oxygen concentration in the Ti samples through their oxide formation reactions. In particular, it was demonstrated that Tm vapor can deoxidize Ti at or below the oxygen concentration of the Ti sponge produced by the Kroll process (∼500 mass ppm O).

Fig. 4 Comparison of the oxygen concentrations in the Ti samples after the deoxidation experiments utilizing Sm metal (Ti-SS in Exp. no.: E-2, Ti-ho in Exp. nos.: AM-1 and AM-2), Tm metal (Ti samples in Exp. nos.: O-1, O-2, AK-1, and AK-2), Yb metal (Ti-SS in Exp. nos.: D-1, F-1, and G-1) and Yb metal with Yb2O3 (Ti-SS in Exp. nos.: D-2, F-2, and G-2) at 1300 K (1027 °C) (cf. Table 5). The error bars show the analyzed error of the oxygen concentrations in the Ti samples.
Titanium (Ti) has excellent corrosion resistance and biocompatibility. The specific strengths of Ti alloys, such as the Ti–6 mass% Al–4 mass% V (Ti–6Al–4V; Ti-64) alloy, are the highest among all metallic materials. In addition to these excellent properties, Ti has its large crustal reserves on earth, and thus Ti and its alloys are expected to be widespread in all industrial fields.
Because the mechanical properties of Ti and its alloys are highly dependent on oxygen (O) impurities, the oxygen concentrations in Ti materials are strictly controlled during the production process of Ti products. However, owing to its high chemical affinity with oxygen, low-productivity and high-cost smelting and fabrication processes are required to produce Ti products with low oxygen concentration from oxide ores. Additionally, the yield of Ti mill products in the fabrication process is extremely low, leading to the generation of a large amount of Ti scrap that is heavily contaminated with oxygen impurities. As a result, Ti products are expensive, and their use is currently limited to high-value-added materials in the aircraft, medical, and chemical plant industries.
Ti scrap that contains relatively low concentrations of impurities such as iron (Fe) and oxygen is reused as a raw material for Ti ingots by melting the scrap together with virgin Ti sponge in the production process for Ti ingots. However, because the oxygen concentration in the Ti melt cannot be decreased in this melting process, the input amount of the scrap of Ti and its alloys with high oxygen concentration must be limited to control the overall oxygen concentration of the produced Ti ingots. Ti scrap with large amounts of impurities, which cannot be reused as a raw material for Ti ingots, is used as a raw material for the production of ferrotitanium (Fe–Ti alloy) and cascaded/recycled into the steelmaking process.1)
As the increasing demand for Ti products for aircraft and other applications increases the production of Ti metal, a large amount of scrap of Ti and its alloys will be generated. Because the Ti smelting process consumes a large amount of energy and generates a large amount of carbon dioxide (CO2), the recycling of Ti scrap is important from the perspectives of economic rationality and environmental impact. Based on this background, in this study, we developed an upgrade recycling process to convert Ti scrap with high oxygen concentration into Ti with low oxygen concentration using a deoxidation technology for Ti with vapor of rare earth (RE) metals.
Various methods have been developed to remove oxygen from Ti,1,2) many of which utilize thermochemical reactions with metal deoxidants or electrochemical reactions in molten salt fluxes.3–21) In contrast, this study aims to develop a deoxidation method that supplies the vapor of the metal deoxidant to Ti scrap. In this method, the vapor of metal deoxidants and fluxes is supplied to Ti scrap as aids for the deoxidation reaction via the gas phase. As a result, the amount of deoxidants and fluxes adhered to the Ti scrap can be reduced, which can simplify the separation and removal of these deposits from Ti scrap.
Previous studies attempted the deoxidation of Ti using calcium (Ca) vapor, and produced Ti with 500 mass ppm O or less through the CaO formation reaction (O (in Ti) + Ca (g) → CaO (s)).22–36) Fisher et al. established a plant for removing oxygen impurities from Ti scrap using Ca vapor.37–40) Although the plant is no longer in industrial operation today, the technique was a pioneering attempt to realize a deoxidation method that supplies the deoxidant to Ti via the gas phase.
In this study, we focused on RE metals, which are expected to exhibit high deoxidation abilities for Ti as the deoxidants. In our previous studies, we developed production methods for Ti with extremely low oxygen concentrations using REs as deoxidants based on the formation reactions of oxides and oxychlorides.10–21,41,42) Although these techniques utilized deoxidation reactions for Ti in contact with condensed phases such as molten salts as reaction media, the deoxidation of Ti using RE vapor has not yet been investigated experimentally.
Figure 1 shows the temperature dependence of the vapor pressures of the REs, Ca, and magnesium (Mg).43,44) Recently, based on thermodynamic consideration, we demonstrated the possibility of producing Ti with 500 mass ppm O or less by utilizing the vapor of REs with high vapor pressures, such as samarium (Sm), europium (Eu), thulium (Tm), and ytterbium (Yb), as deoxidants through the formation reactions for their oxides and oxyhalides.45) In this study, we studied a novel deoxidation process for Ti based on supplying the vapor of Sm, Eu, Tm, and Yb as deoxidants to Ti scrap via the gas phase.
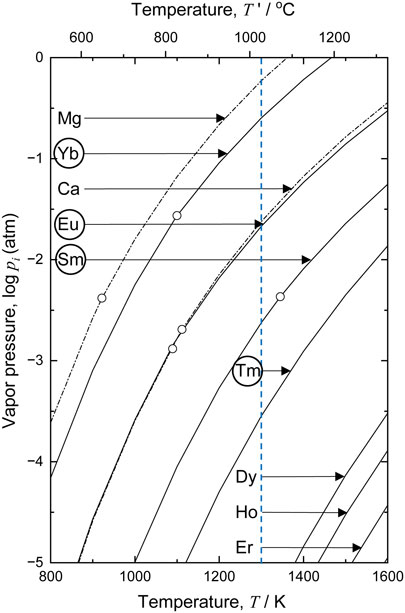
Conventionally, because of the high prices of REs compared to Ti, the utilization of REs as deoxidants for Ti scrap has been considered unreasonable from an economic perspective. However, with the recent increase in the production of some REs such as neodymium (Nd) and dysprosium (Dy) resulting from the increase in demand for RE magnets, other REs produced as by-products are in oversupply. In the future, price of these by-product REs may decrease, and these REs will be utilized for deoxidation of Ti with reasonable cost. Although Sm, Eu, Tm, and Yb are currently used in some applications, as listed in Table 1, major applications of Tm and Yb have not been established yet. Therefore, it is important to develop uses for these REs from the perspective of resource utilization.46)

The new Ti deoxidation technology in this study is expected to not only promote the recycling of Ti scrap but also enable the development of a new application for REs.
The $\beta$-Ti phase is a stable phase of Ti at high temperature, and the thermodynamic relationship between the concentration of oxygen dissolved in solid $\beta$-Ti phase ([O]Ti (mass%)) and the partial pressure of oxygen (O2) gas in the system ($p_{\text{O}_{2}}$ (atm)) can be expressed by eqs. (1)–(3):3)
| \begin{equation} \text{1/2 O$_{2}$ ($g$)} = \text{O (1$\,$mass%, in $\beta$-Ti)} \end{equation} | (1) |
| \begin{equation} \Delta G_{\text{1,$\,$Ti}}^{\circ} = -2.303 \cdot \text{R}T\log\frac{f_{\text{O}} \cdot [\text{O}]_{\text{Ti}}}{p_{\text{O${_{2}}$}}^{1/2}} \end{equation} | (2) |
| \begin{equation} = -583000 + \text{88.5 $T$ (J${\cdot}$mol$^{-1}$) [1173–1373$\,$K]} \end{equation} | (3) |
The deoxidation reaction of $\beta$-Ti using a RE metal as the deoxidant through the formation of the RE oxide and oxyhalide is expressed by the thermodynamic relations in eqs. (4)–(6) and (7)–(8), respectively.
| \begin{align} &\text{O (1$\,$mass%, in $\beta$-Ti)} + \text{2/3 RE ($s$, $l$)} \\ &\quad= \text{1/3 RE$_{2}$O$_{3}$ ($s$)} \end{align} | (4) |
| \begin{equation} \Delta G_{\text{deox,$\,$RE${_{2}}$O${_{3}}$}}^{\circ} = 1/3\Delta G_{\text{f,$\,$RE${_{2}}$O${_{3}}$}}^{\circ} - \Delta G_{\text{1,$\,$Ti}}^{\circ} \end{equation} | (5) |
| \begin{equation} \Delta G_{\text{deox,$\,$RE${_{2}}$O${_{3}}$}}^{\circ} = -2.303 \cdot \text{R}T\log\frac{a_{\text{RE${_{2}}$O${_{3}}$}}{}^{1/3}}{f_{\text{O}} \cdot [\text{O}]_{\text{Ti}} \cdot a_{\text{RE}}{}^{2/3}} \end{equation} | (6) |
| \begin{align} &\text{O (1$\,$mass%, in $\beta$-Ti)} + \text{2/3 RE ($s$, $l$)} \\ &\quad + \text{1/3 RECl$_{3}$ ($s$, $l$)} = \text{REOCl ($s$)} \end{align} | (7) |
| \begin{equation} \Delta G_{\text{deox,$\,$REOCl}}^{\circ} = \Delta G_{\text{f,$\,$REOCl}}^{\circ} - 1/3\Delta G_{\text{f,$\,$RECl${_{3}}$}}^{\circ} - \Delta G_{\text{1,$\,$Ti}}^{\circ} \end{equation} | (8) |
| \begin{align} &\Delta G_{\text{deox,$\,$REOCl}}^{\circ}\\ &\quad = -2.303 \cdot \text{R}T\log\frac{a_{\text{REOCl}}}{f_{\text{O}} \cdot [\text{O}]_{\text{Ti}} \cdot a_{\text{RE}}{}^{2/3} \cdot a_{\text{RECl${_{3}}$}}{}^{1/3}} \end{align} | (9) |
To calculate the oxygen concentrations in $\beta$-Ti under the deoxidation equilibria, the values of the standard Gibbs energy changes of the deoxidation reactions at the deoxidation temperature are required. However, there are few reliable reports on thermodynamic data for RE compounds at high temperatures.
In this study, we experimentally investigated the feasibility of a technique that supplies REs with high vapor pressure to Ti via the gas phase and deoxidizes Ti through the formation of RE oxides.
Table 2 lists the calculated oxygen partial pressure ($p_{\text{O}_{2}}$), calculated equilibrium oxygen concentration in $\beta$-Ti (CO, calc.), experimentally determined equilibrium oxygen concentration in $\beta$-Ti (CO, exp.) under the RE/REOx equilibrium, and calculated vapor pressures of REs (pi) at 1300 K (1027 °C).
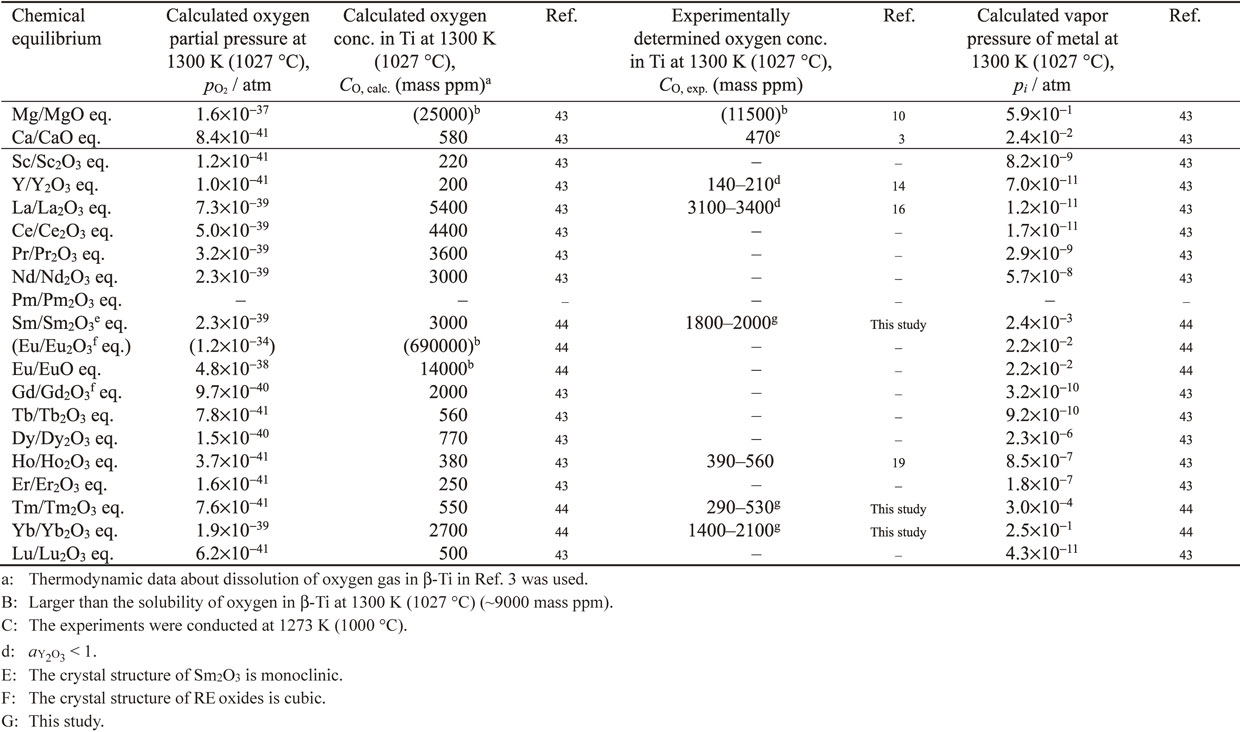
Figure 2 shows the equilibrium oxygen concentrations in $\beta$-Ti under Sm/Sm2O3, Eu/EuO, Tm/Tm2O3, and Yb/Yb2O3 equilibria (CO, calc. (mass ppm) and [O]Ti (mass%)), which were calculated using eq. (6) based on reported thermodynamic data. The oxygen concentrations under the Ca/CaO and Mg/MgO equilibria are also shown for comparison. Each of the RE/REOx equilibria is expected to produce Ti with a lower oxygen concentration than the Mg/MgO equilibrium. In particular, the Tm/Tm2O3 equilibrium can produce Ti with an extremely low oxygen concentration, which is lower than that achieved under the Ca/CaO equilibrium.
Based on the above consideration, we focused on the deoxidation of Ti using the formation reaction of REOx, as shown in eq. (4), and demonstrated a new deoxidation process for Ti that supplies REs with high vapor pressure to Ti via the gas phase.
Figure 3 shows a schematic diagram of the reaction vessel used for the deoxidation experiments. Tables 3 and 4 present detailed information regarding the experimental samples and their initial amounts, respectively. The Ti samples (“Ti-ho”, “Ti-SS”, “Ti-1.2U”, “Ti-2.0L”, and “Ti-6n”), Ti-64 alloy samples (“Ti-ho-64” and “Ti-1.2-64”), Ti crucibles, Ti caps, and Ti foils were cleaned in the order of deionized water, isopropanol (99.7%, Kanto Chemical Co., Inc.), and acetone (99.0%, Kanto Chemical Co., Inc.). The cleaned Ti and Ti-64 alloy samples were further chemically treated with a mixture solution of hydrofluoric acid (46.0–48.0%, Kanto Chemical Co., Inc.), nitric acid (60–61%, Kanto Chemical Co., Inc.), and deionized water (volumetric ratio 1:4:10), and then rinsed with deionized water and isopropanol. The RE metal deoxidants were placed at the bottom of the Ti crucibles. The Ti foils were fixed on the upper side of the crucibles, on which the Ti and Ti-64 alloy samples were placed without physical contact with the deoxidant (the gas-phase side). In the experiments using Eu and Yb as deoxidants, which have lower melting points than the experimental temperature of 1300 K (1027 °C), the Ti samples were immersed in molten Eu or Yb at the bottom of the Ti crucibles (the condensed-phase side). In some experiments using Yb metal, Yb2O3 was also placed at the bottom of the crucibles.

Schematic illustration of the deoxidation experiments utilizing RE metals at 1300 K (1027 °C) (RE: Sm, Eu, Tm, Yb). Yb2O3 was placed at the bottom of the Ti crucibles in Exp. nos.: D-2, F-2, and G-2.

These Ti crucibles were placed in a stainless-steel vessel (SUS316 container) with Ti sponge as a getter for oxygen, nitrogen (N), and moisture, and sealed using tungsten inert gas (TIG) welding. The SUS316 container was maintained at 1300 K (1027 °C) for 173 ks (2 days) in a box-type electric furnace. The diffusion of oxygen in Ti is the rate limiting step for the deoxidation of solid Ti. The reaction time was determined based on the diffusion coefficient of O in $\beta$-Ti at high temperatures,47) the shapes of the Ti samples, and the assumption that a homogeneous distribution of O dissolved in the Ti samples. It was experimentally confirmed in the previous studies that reaction time (∼2 days) is enough for deoxidation of the samples with few mm thickness at 1300 K.3,4,10,14) After the prescribed holding time, the SUS316 container was quenched in water. The SUS316 container and Ti crucibles were cut, and the Ti and Ti-64 alloy samples were extracted. The Ti and Ti-64 alloy samples were washed in tap water and acetic acid to remove the deoxidants, salts, and deoxidation products adhered to the samples. These Ti and Ti-64 alloy samples were cleaned with the above mixture solution of hydrofluoric acid, nitric acid, and deionized water, and then washed with deionized water and isopropanol. The oxygen and nitrogen concentrations in the Ti and Ti-64 alloy samples were measured using the inert gas fusion method (TC-600; LECO Corporation). The crystal phases of the deoxidation products in the Ti crucibles were identified using X-ray diffraction (XRD; D2 Phaser, Bruker). For the experiments using Sm and Yb as deoxidants, the mixtures of the deoxidants and deoxidation products in the Ti crucibles were analyzed. For the experiments using Tm as the deoxidant, the deoxidation products formed on the back surface of the Ti cap inside the Ti crucible were analyzed.
Table 5 summarizes the oxygen and nitrogen concentrations in the Ti and Ti-64 alloy samples before and after the deoxidation experiments. The oxygen concentrations of the Ti-64 alloy samples after deoxidation were lower than those of the Ti samples after deoxidation. In this study, we mainly discuss the oxygen concentrations in the Ti samples. Figure 4 shows the oxygen concentrations in the Ti samples (Ti-ho and Ti-SS) after the deoxidation experiments.

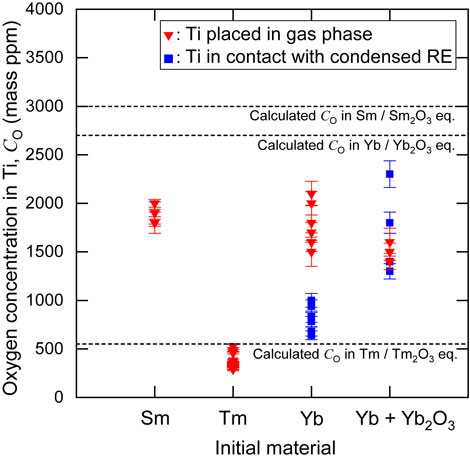
Comparison of the oxygen concentrations in the Ti samples after the deoxidation experiments utilizing Sm metal (Ti-SS in Exp. no.: E-2, Ti-ho in Exp. nos.: AM-1 and AM-2), Tm metal (Ti samples in Exp. nos.: O-1, O-2, AK-1, and AK-2), Yb metal (Ti-SS in Exp. nos.: D-1, F-1, and G-1) and Yb metal with Yb2O3 (Ti-SS in Exp. nos.: D-2, F-2, and G-2) at 1300 K (1027 °C) (cf. Table 5). The error bars show the analyzed error of the oxygen concentrations in the Ti samples.
In the experiments using Sm as the deoxidant (Exp. nos.: E-2, AM-1, and AM-2), the Ti samples initially containing high concentrations of oxygen (Ti-ho and Ti-SS) were deoxidized to low oxygen concentrations, although the oxygen concentrations in the Ti samples containing initially low concentrations of oxygen (Ti-1.2U and Ti-2.0L) increased. The oxygen concentration under the equilibrium between Sm and its oxide is expected to be the value between these oxygen concentrations after the experiments. There were few oxygen sources to increase oxygen concentration in the Ti samples such as air contained initially in SUS316 container and oxygen contained in the deoxidants and their oxides, etc. Because there was little oxygen source to increase the oxygen concentration in the Ti samples placed on the gas-phase side, it is considered that the oxygen concentrations in the Ti samples containing initially low concentrations of oxygen did not reach the equilibrium value after the experiments. Therefore, in this study, the oxygen concentrations in the Ti samples initially containing high concentrations of oxygen after the experiments were regarded as the oxygen concentration in Ti under the equilibrium between Sm and its oxide. The oxygen concentrations in the Ti samples initially containing high concentrations of oxygen and the Ti-64 alloy samples (Ti-ho-64 and Ti-1.2-64) after the experiments were 1800–2000 mass ppm O (maximum analysis error: 9%) and 730–910 mass ppm O (maximum analysis error: 9%), respectively.
In the experiments using Eu as the deoxidant (Exp. nos.: P-1, P-2, AL-1, and AL-2), the oxygen concentrations in almost all of the Ti and Ti-64 alloy samples increased. However, owing to the large scatter in the experimental results and the large deviation from the thermodynamically calculated value (cf. Table 2), it was difficult to evaluate the equilibrium oxygen concentration in Ti under the equilibrium between Eu and its oxide in this study.
In the experiments using Tm as the deoxidant (Exp. nos.: O-1, O-2, AK-1, and AK-2), the Ti and Ti-64 alloy samples were deoxidized to 290–530 mass ppm O (maximum analysis error: 9%) and 150–240 mass ppm O (maximum analysis error: 9%), respectively. As shown in Fig. 1 and Table 2, the vapor pressure of Tm is 3.0 × 10−4 atm at 1300 K (1027 °C), which is lower than those of Sm, Eu, and Yb. However, the large decreases in the oxygen concentrations in the Ti and Ti-64 alloy samples after the deoxidation experiments demonstrates that Tm can be supplied to Ti via the gas phase, and that the Tm vapor can deoxidize Ti.
In some of the experiments using Yb as the deoxidant (Exp. nos.: D-1, F-1, and G-1), only Yb metal was installed with the Ti samples in the Ti crucibles. In other experiments (Exp. nos.: D-2, F-2, and G-2), Yb metal and Yb2O3 were installed together in the Ti crucibles. The oxygen concentrations in the Ti samples initially containing high concentrations of oxygen (T-SS) decreased to 640–1000 mass ppm O (maximum analytical error: 9%) on the condensed-phase side after the experiments in which only Yb metal was installed in the Ti crucibles. However, the oxygen concentrations decreased to 1300–2300 mass ppm O (maximum analytical error: 9%) after the experiments in which both Yb metal and Yb2O3 were installed in the Ti crucibles. One possible reason for the lower oxygen concentration in the Ti samples treated under the system without Yb2O3 input is that the small amount of Yb oxide formed as a deoxidation product entirely dissolved in the molten Yb melt, leading to a decrease in the activity of the oxide. As in the experiments using Sm, the oxygen concentrations of the Ti samples placed on the gas-phase side showed large discrepancies resulting from the initial oxygen concentrations. The oxygen concentrations of the Ti samples initially containing high concentrations of oxygen (Ti-SS) placed on the gas-phase side after the experiments were regarded as the oxygen concentration in Ti under the equilibrium between Yb and its oxide. The oxygen concentrations of the Ti samples initially containing high concentrations of oxygen (Ti-SS) and the Ti-64 alloy samples (Ti-1.2-64) placed on the gas-phase side after the experiments were 1400–2100 mass ppm O (maximum analysis error: 10%) and 550–730 mass ppm O (maximum analysis error: 9%), respectively.
The oxygen concentrations in the Ti samples after deoxidation with Sm, Tm, and Yb as deoxidants were lower than the calculated values estimated from previously reported thermodynamic data.
As can be seen from the data in Table 5, the nitrogen concentration in the Ti samples tends to increase. Titanium has a high affinity for nitrogen. Therefore, when rare earth metals are used as deoxidants for Ti, the nitrogen in rare earth metals can be a source of nitrogen contamination in Ti. On the other hand, after deoxidation, the concentration of nitrogen in Ti samples installed in the gas phase, which were not in direct contact with rare earth metals, tends to be significantly lower than those in direct contact with rare earth metals. The deoxidation method used in this study, in which rare earth vapors are supplied to the Ti sample surface via the gas phase, is effective for preventing nitrogen contamination of Ti scrap.
4.2 Identification of deoxidation productsFigure 5 shows the XRD results for the deoxidant and deoxidation products after the experiments. Figure 5(a) shows the XRD pattern of the mixture of Sm metal and deoxidation product after the experiment using Sm (Exp. no.: AM-1). The results indicate the presence of trivalent Sm oxide, Sm2O3, as the deoxidation product. Figure 5(b) shows the XRD pattern of the back surface of the Ti cap placed on the upper side of the Ti crucible (inside the Ti crucible) after the experiment using Tm (Exp. no.: AK-2). The results indicate the presence of trivalent Tm oxide, Tm2O3, as the deoxidation product. Figure 5(c) shows the XRD pattern of the mixture of Yb metal and the deoxidation product after the experiment in which Yb metal and Yb2O3 were installed together (Exp. no.: D-2). The results indicate the presence of trivalent Yb oxide, Yb2O3, as the deoxidation product. In all cases, low-valent oxides, such as SmO, TmO, and YbO, were not observed. Therefore, in the experiments using Sm, Tm, and Yb as deoxidants, the Sm/Sm2O3, Tm/Tm2O3, and Yb/Yb2O3 equilibria were established, respectively.
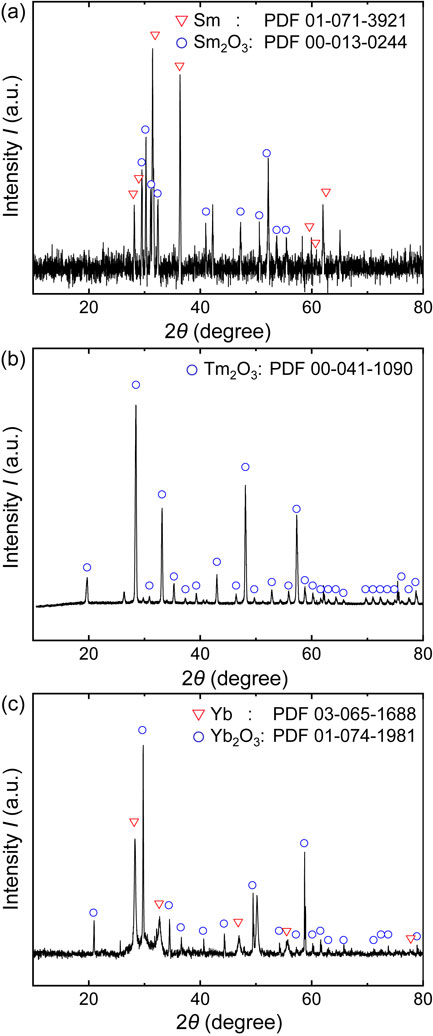
XRD patterns of the products in the deoxidation experiments: (a) mixture of Sm metal and a deoxidation product after the deoxidation experiment using Sm metal (Exp. no.: AM-1), (b) deposit on the surface of the back side of the Ti cap after the deoxidation experiment using Tm metal (Exp. no.: AK-2), and (c) mixture of Yb metal and a deoxidation product after the deoxidation experiment using Yb metal (Exp. no.: D-2).
Much of the reported thermodynamic data for RE oxides may large errors. We calculated the standard Gibbs energy of formation of RE2O3 ($\Delta G_{\text{f},\,\text{RE}_{2}\text{O}_{3}}^{\circ}$) using the oxygen concentrations in the Ti samples obtained from the deoxidation experiments. Equation (10) below is derived from eqs. (5) and (6):
| \begin{align} &\Delta G_{\text{f,$\,$RE${_{2}}$O${_{3}}$}}^{\circ} \\ &\quad = 3\left(\Delta G_{\text{1,$\,$Ti}}^{\circ} - 2.303 \cdot \text{R}T\log\frac{a_{\text{RE${_{2}}$O${_{3}}$}}{}^{1/3}}{f_{\text{O}} \cdot [\text{O}]_{\text{Ti}} \cdot a_{\text{RE}}{}^{2/3}}\right) \end{align} | (10) |
In the previous study, through the analysis using the standard enthalpy of formation of RE2O3 at 298 K (25 °C), $\Delta H_{\text{f},\,\text{RE}_{2}\text{O}_{3},\,298\text{K}}^{\circ}$, we predicted that the deoxidation abilities of REs for Ti are high in the order of Tm, Sm, Yb, and Eu (Tm > Sm > Yb > Eu).45) See the reference for details. The results of this study provide an example that validates the prediction of the deoxidation abilities of REs for Ti based on our thermodynamic consideration in the Ref. 45).
In this study, we experimentally demonstrated a new deoxidation method in which RE metals with high vapor pressures, such as Sm, Eu, Tm, and Yb, were supplied as deoxidants to Ti and Ti-64 alloy samples via the gas phase, and oxygen was removed from these Ti samples. It was confirmed that in experiments using Sm, Tm, and Yb as deoxidants for Ti, no low-valent oxides such as SmO, TmO, and YbO were formed as deoxidation products, and that the Sm/Sm2O3, Tm/Tm2O3, and Yb/Yb2O3 equilibria determined the oxygen potentials in the systems. The obtained oxygen concentrations in Ti were lower than those estimated based on the previously reported thermodynamic data. The equilibrium oxygen concentrations in the Ti-64 alloy samples were lower than those in the Ti samples. These results demonstrate that this new deoxidation method, which supplies RE metals with high vapor pressure as deoxidants to Ti via the gas phase, can be an effective process for oxygen removal from scrap of Ti and its alloys.
We would like to thank Mr. Koichi Hirota at Shin-Etsu Chemical Co., Ltd. for providing us with rare earth metal samples. We are grateful to Mr. Chihiro Taki at Toho Technical Service Co., Ltd. and Mr. Nobuo Fukada of Toho Titanium Co., Ltd. for their cooperation in the preparation of the Ti and Ti alloy samples with high oxygen concentrations. We would like to thank graduate student Dr. Akihiro Iizuka of the Department of Materials Engineering, Graduate School of Engineering, the University of Tokyo, for his experimental support and comments on this manuscript. This work was financially supported by the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Scientific Research (S) (KAKENHI Grant No. 19H05623). This paper is also based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO), Feasibility Study Program (Uncharted Territory Challenge 2050).