2023 年 64 巻 2 号 p. 568-577
2023 年 64 巻 2 号 p. 568-577
To ascertain the effect of solution pH of Na2MoO4 chemical conversion treatment for aluminum/steel joints on corrosion resistance, AA5083 aluminum alloy and AISI 1045 carbon steel were immersed in 50 mM Na2MoO4 at pH ranges of 8–12 under galvanically coupled condition. Subsequently, in diluted synthetic seawater, the galvanic corrosion resistance of the AA5083 alloy connected to the AISI 1045 carbon steel was assessed. The number of localized corrosion damages was counted, and AA5083 treated at pH 11 was found to be the better corrosion resistance. The oxygen reduction current on bulk Al6(Fe, Mn) decreased with increasing solution pH of the conversion treatment. The Al6(Fe, Mn) particles on AA5083 were not preferential cathodes, and alkalization through oxygen reduction would not occur when the treatment was performed above pH 9. Auger electron spectroscopy analysis showed that Mo-accumulation, Fe-removal, and film thickening occurred on the particles of AA5083 treated at pH 11. These factors contributed to the suppression of the cathodic activity of the Al6(Fe, Mn) particles, resulting in the improved galvanic corrosion resistance of AA5083.

Fig. 8 Effect of solution pH used in the conversion treatment on the number of localized corrosion damages on as-polished and conversion-treated AA5083 after galvanic current and potential measurements as shown in Fig. 6.
To achieve a carbon-neutral society, the decrease in CO2 gas emissions from automobiles, which has been a major problem,1,2) must be addressed. Using thinner steel plates with high strength for automobile bodies is a strategy commonly employed to solve this problem.3) Additionally, multi-material structures that combine steels and light materials are attracting attention.4–6) Steel/aluminum joints are effective and realistic proposition because of their high durability and low costs.7) However, there are several technical challenges to their realization from welding,8) recycling,9) and corrosion engineering.10–14) In multi-material structures under humidified conditions, galvanic corrosion is an expected serious problem. In the case of dissimilar materials joint between a steel and an Al alloy, the initiation and growth of corrosion on the Al alloy are enhanced by the increase in the electrode potential of the Al alloy by galvanic coupling to the steel.15–18)
Corrosion of automobile bodies is generally classified into localized corrosion in chloride solutions around neutral pH. This is because airborne chlorides and de-icing salts form chloride solutions because of rainfall and condensation. In chloride solutions, intermetallic particles (IMPs) on Al alloys play an important role in pitting and filiform corrosion.19–22) It is known that Fe- or/and Cu-containing IMPs shows higher electrode potential compared with the Al-matrix of Al alloys. Therefore, the oxygen reduction reaction preferentially occurred on the IMPs, and the pH on and around the IMPs increased. As a result, alkaline dissolution of the Al-matrix around the IMPs occurs, and trenches are generated along their periphery. The trenches are known to be the initiation sites for localized corrosion. Kakinuma et al. performed in situ observations and micro-scale electrochemical measurements in 0.1 M NaCl and demonstrated that the morphological change from trenching to pitting occurs around Fe-containing IMPs on AA1050.19) Yasakau et al. studied pit initiation in AA5083 using scanning Kelvin force probe microscopy.23) They also proposed that Fe-rich IMPs act as cathodes, and pit initiation and growth occur around the IMPs because of alkalization by the oxygen reduction reaction. Even under galvanic coupling of an Al alloy and a steel, the reactivity of the oxygen reduction reaction on IMPs and the alkaline dissolution of the Al-matrix plays important roles in the initiation of localized corrosion on Al alloys.24–26) Charles-Granville et al. analyzed the galvanic corrosion behavior of AA7050-T7451 coupled to Type 316 stainless steel in Cl−-containing solutions.24) They reported that there is a possibility that the Cu-rich IMPs in AA7050 become initiation sites for pitting. In our previous study, Al6(Fe, Mn) particles on AA5083 were found to be the initiation sites of filiform corrosion when AA5083 was coupled to pure Fe in diluted synthetic seawater.25) Therefore, suppression of the oxygen reduction reaction on IMP and alkaline dissolution around IMP are required to prevent galvanic corrosion of Al alloys. These strategies are the same as those of improving corrosion resistance of Al alloys under uncoupled condition.
Chemical conversion treatments have been successfully adopted for Al alloys to suppress the cathodic activity on the IMPs and alkalization.26–30) Shruthi and Swain evaluated the effect of trivalent chromium treatment on the oxygen reduction reaction rate on AA2024-T3.26) They reported that the oxygen reduction reaction on the Cu-rich IMPs was extremely suppressed by the treatment. For Al alloys, Zr-based conversion treatments have been extensively studied.31–34) Cerezo et al. analyzed the composition of the conversion film on AA6014 after conversion treatment in a H2ZrF6 solution.31) Their research suggested that ZrO2 conversion layer improves corrosion resistance. Conversion treatment using Na2MoO4 solutions has also been actively studied because Na2MoO4 is an environmentally-friendly species.35–41) Lopez-Garrity and Frankel studied the corrosion inhibition of AA2024-T3 treated in 0.1 M NaCl–Na2MoO4 solutions.37) They proposed that the surface of IMPs was covered with a Mo-based oxide, which reduced cathodic activity and did not cause alkalization on and around the IMPs.
For the practical use of multi-material structures, it is necessary to apply the conversion treatment to Al alloy/steel joints rather than to individual materials. In the case of Al alloy/steel joints, an Al alloy and a steel are galvanically connected to each other in a conversion treatment solution, and it is difficult to obtain satisfactory performance for both materials. To the best of our knowledge, there are no reports of optimal conditions for Al alloy/steel joints in MoO4− conversion treatment. In this study, in the conversion treatment, AA5083 aluminum alloy and AISI 1045 carbon steel were immersed in a Na2MoO4 solution under galvanically coupled condition. Subsequently, in diluted synthetic seawater, the galvanic corrosion resistance of the AA5083 alloy coupled to the AISI 1045 carbon steel was evaluated. The solution pH of the chemical conversion treatment was varied in the pH ranges of 8–12 because MoO4− is possible to be readily reduced to Mo-oxides and/or Mo-hydroxide in alkaline environments according to Pourbaix diagrams of the Mo–H2O system.42) Electrochemical measurements and surface analysis were performed, and the role of conversion treatment in the galvanic corrosion resistance of AA5083 alloy coupled to AISI 1045 carbon steel is clarified in terms of the oxygen reduction reaction on IMPs and the alkaline dissolution around IMPs.
As specimens, AA5083 aluminum alloy, AISI 1045 carbon steel, and pure Al (99.999 mass%) were used. Tables 1 and 2 list the chemical compositions of AA5083 and AISI 1045 carbon steels, respectively. A sheet of 3 mm-thick AA5083 was annealed at 723 K for 90 min (water-quenched) to suppress intergranular corrosion due to β phase (Al3Mg2) precipitation at grain boundaries. A sheet of 3 mm-thick AISI 1045 carbon steel was austenitized at 1123 K for 60 min and then water-quenched to obtain a full martensite structure. Then, tempering was carried out at 873 K for 6 min (water-quenched). The heat treatment of AISI 1045 carbon steel was conducted in an Ar atmosphere to prevent decarburization.43) Pure Al (1 mm in thickness) was used without any heat treatment. Sheets of AA5083 alloy, AISI 1045 carbon steel, and pure Al were cut into 15 mm × 25 mm pieces. The surfaces of AA5083 and pure Al specimens were polished to 0.25 µm with a diamond suspension. The AISI 1045 carbon steel was polished to 1 µm with a diamond suspension. After polishing, the specimens were cleaned using ethanol.

A 30 g ingot of Al6(Fe, Mn) was fabricated by arc melting in a Ti-gettered Ar atmosphere to estimate the polarization behavior of Al6(Fe, Mn) particles on AA5083. The ingot was annealed at 923 K for 72 h and cut into 10 mm × 15 mm × 5 mm pieces. In this study, these pieces were referred to as bulk Al6(Fe, Mn). Bulk Al6(Fe, Mn) specimens were polished to 0.25 µm with a diamond suspension using ethanol as a lubricant. Finally, the specimens were cleaned with ethanol.
2.2 Electrochemical measurementsFor the electrochemical measurements, diluted synthetic seawater (pH 8.2, adjusted with NaOH) was used to simulate corrosion in marine atmospheric environments. According to the ASTM D1141 standard, synthetic seawater (pH 8.2) was prepared and diluted by a factor of 100 with deionized water. The chloride ion concentration in this diluted solution was calculated to be 5.64 × 10−3 M (200 mg L−1). Before conducting the electrochemical measurements, the pH of the diluted synthetic seawater was readjusted using NaOH.
Potentiodynamic polarization curves were measured in naturally aerated diluted synthetic seawater at 298 K. The working electrode area of the AA5083 alloy and AISI 1045 carbon steel was 10 mm × 10 mm. For bulk Al6(Fe, Mn), an area of 100 µm × 100 µm was used as a working electrode to avoid casting defects. After polarization, the area was precisely measured, and the current density was calculated. The scan rate of the electrode potential was set to 3.8 × 10−4 V s−1 (23 mV min−1). All potentials refer to the Ag/AgCl (3.33 M KCl) electrode (0.206 V vs. standard hydrogen electrode at 298 K).
The galvanic corrosion resistance of the AA5083 alloy connected to AISI 1045 carbon steel was assessed in naturally aerated diluted synthetic seawater at 298 K. The galvanic coupling current between the AA5083 alloy and the AISI 1045 carbon steel specimens was monitored using a zero-resistance ammeter. The electrode area was 10 mm × 10 mm. The distance between the two electrodes was maintained at 10 mm. In this study, the polarity of the galvanic current was denoted by the direction of the current flowing through AA5083. The electrode potential of AA5083 was also measured. After galvanic current and potential measurements, the number of localized corrosion damages was counted using an optical microscope. Pitting corrosion is defined as corrosion damage with a diameter larger than 100 µm, while filiform corrosion is defined as thread-like corrosion damage with a length of less than 100 µm.
To assess the alkaline dissolution resistance of a surface film on Al-matrix of AA5083, the effect of pH on the open-circuit potential of the pure Al was measured in the naturally aerated diluted synthetic seawater at 298 K. The electrode area was 10 mm × 10 mm. The pH of the solution was increased stepwise from 9 to 14 using NaOH. At each pH value, the open-circuit potential was measured for 600 s.
2.3 Chemical conversion treatmentsAA5083 alloy and AISI 1045 carbon steel were connected via a zero-resistance ammeter and immersed in 50 mM Na2MoO4 as a chemical conversion treatment under naturally aerated conditions. The pH of the solution was varied from 8 to 12 using NaOH. The size of the electrode areas was 1.2 mm × 1.2 mm. The distance between the two electrodes was kept at 10 mm. The immersion time was 10 min and the temperature was maintained at 298 K. In this study, unless otherwise noted, conversion treatment is the treatment under galvanically coupled conditions. As a reference, conversion treatment under uncoupled conditions was performed at 298 K in 50 mM Na2MoO4 (pH 8.0) for AA5083 alloy and AISI 1045 carbon steel separately. Moreover, potentiostatic polarization was performed at 298 K for pure Al and bulk Al6(Fe, Mn) specimens in 50 mM Na2MoO4 (pH ranges of 8–11). The polarization potentials corresponded to the electrode potentials under galvanically coupled condition in the conversion treatment: −0.30 V at pH 8, −0.30 V at pH 9, −0.40 V at pH 10, and −0.80 V at pH 11. After the conversion treatment, the specimens were rinsed with deionized water and flowed by a stream of N2 gas.
2.4 Surface observations and analysisAfter electrochemical measurements, the specimens were rinsed with deionized water and ethanol. Surface observations were performed using an optical microscope. The compositions of surface oxide films on Al-matrix and Al6(Fe, Mn) were analyzed by Auger electron spectroscopy (AES). The specimens were tilted to 30° and sputtered by Ar+. The sputtering rate was calculated to be 1.13 nm min−1 based on the thermally oxidized SiO2/Si. The sputtering area was 2 µm × 2 µm.
In the first step, a chemical conversion treatment was performed under uncoupled conditions. Figure 1(a) shows the time variation in the open-circuit potential (OCP) of the AA5083 alloy and AISI 1045 carbon steel in 50 mM Na2MoO4 at pH 8. Since Na2MoO4 chemical conversion treatment is usually performed at pH 8,37) the conversion treatment under uncoupled condition was conducted at this pH. The OCP of AA5083 was initially −1.30 V and increased to −1.0 V. In contrast, the OCP of AISI 1045 carbon steel was stable at −0.15 V. Figures 1(b) and 1(c) show optical micrographs of AA5083 alloy and AISI 1045 carbon steel after conversion treatment. The surface of AA5083 was discolored to blue, and small brown dots appeared. These dots correspond to intermetallic particles. The blue discoloration on the Al-matrix is expected to be due to Mo-oxide and/or Mo-hydroxide formation.42) On contrary, no apparent discoloration was observed on AISI 1045 carbon steel.

Time variation in (a) open-circuit potential of AA5083 alloy and AISI 1045 carbon steel during conversion treatment uncoupled condition in 50 mM Na2MoO4 at pH 8 (298 K). Optical micrographs of (b) AA5083 alloy and (c) AISI 1045 carbon steel after treatment shown in (a).
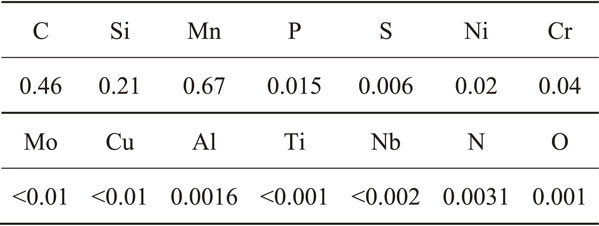
In the diluted synthetic seawater, the effect of conversion treatment on the galvanic corrosion resistance of AA5083 alloy coupled to AISI 1045 carbon steel was evaluated. Figures 2(a) and 2(b) show the time variations in the galvanic current density and electrode potential of the conversion-treated AA5083 alloy coupled to the conversion-treated AISI 1045 carbon steel. Each specimen was treated under uncoupled conditions at pH 8 shown in Fig. 1. For the galvanic current, an anodic current was observed on AA5083. Both of the galvanic current density and electrode potential decrease with time. After the measurements, only one corrosion damage was observed in AA5083, as shown in Fig. 2(c). On the specimens without chemical conversion treatment, many pits and filiform corrosion damages have been reported to occur.25) Thus, it was found that the galvanic coupling between the separately conversion-treated AA5083 alloy and AISI 1045 carbon steel specimens provides high corrosion resistance.
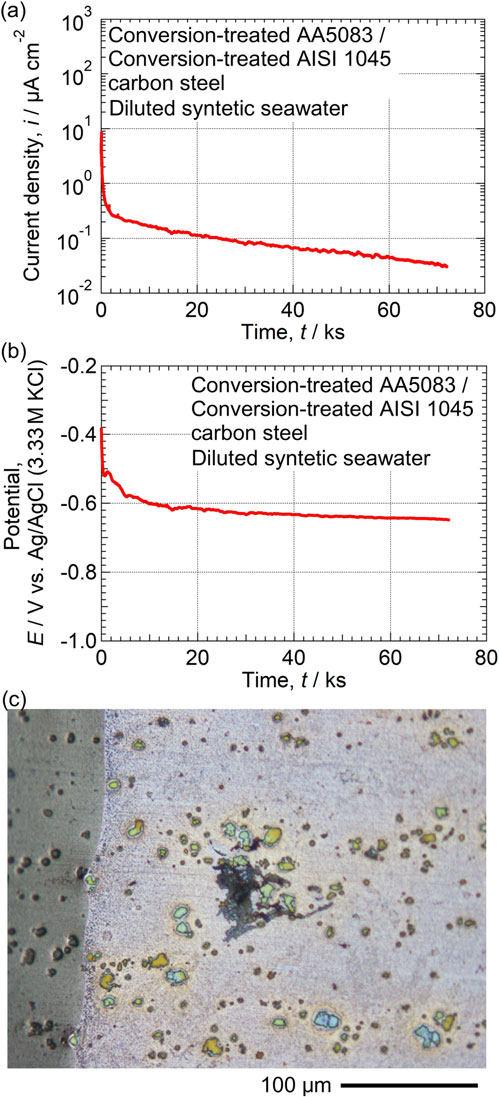
Time variations in (a) galvanic current density and (b) electrode potential of conversion-treated AA5083 alloy coupled to conversion-treated AISI 1045 carbon steel in the naturally aerated diluted synthetic seawater at pH 8.2 (298 K). Each specimen was conversion-treated under uncoupled condition shown in Fig. 1. (c) Optical micrographs of the AA5083 after the galvanic current and potential measurements.
The AA5083 alloy and AISI 1045 carbon steel were connected via a zero-resistance ammeter and immersed in 50 mM Na2MoO4 at various pH values to simulate the chemical conversion treatment of aluminum/steel joints. Figure 3 shows the effect of pH on galvanic current densities and electrode potentials on the AA5083 during conversion treatment. In all the cases, the anodic current was measured for AA5083. As shown in Fig. 3(a), in the initial period, the galvanic current densities were similar at pH ranges of 8 to 11, but the decrease in current densities became small with increasing pH. At pH 11, almost no decrease was observed. At pH 12, the current density was approximately one order of magnitude higher than that at pH 11. For the electrode potentials, the time variations were almost the same at pH ranges 8–10. At pH 11, the potential became unstable and gradually decreased over time. At pH 12, the potential stabilized but decreased to −1.18 V.

Time variations in (a) galvanic current densities and (b) electrode potentials of AA5083 coupled to AISI 1045 carbon steel in 50 mM Na2MoO4 at pH 8, 9, 10, 11, and 12.
Figures 4 and 5 show optical micrographs of the AA5083 alloy and AISI 1045 carbon steel after the conversion treatment, as shown in Fig. 3. As a reference, a surface image under the as-polished condition is presented. In Fig. 4, the small black dots correspond to IMPs such as Al6(Fe, Mn). In the pH ranges of 8–11, the number of black dots increased with increasing pH, but no discoloration of the Al-matrix was observed. However, at pH 12, the significant corrosion occurred on the surface. This was likely associated with the larger current density at pH 12, as shown in Fig. 3(a). Comparing Figs. 4 and 1(b), the surface color of AA5083 was different when AA5083 was connected to steel and AA5083 was not connected to steel. In the conversion treatment of AA5083 under galvanically coupled to the steel, the electrode potential of AA5083 increased, which may have inhibited the surface film formation of Mo-oxide and/or Mo-hydroxide on AA5083.

Optical micrographs of (a) as-polished AA5083 and (b)–(f) conversion-treated AA5083 under galvanically coupled with AISI 1045 carbon steel. The conversion treatment was performed in 50 mM Na2MoO4 at (b) pH 8, (c) pH 9, (d) pH 10, (e) pH 11, (f) pH 12.

Optical micrographs of (a) as-polished AISI 1045 carbon steel and (b)–(f) conversion-treated AISI 1045 carbon steel under galvanically coupled with AA5083. The conversion treatment was performed in 50 mM Na2MoO4 at (b) pH 8, (c) pH 9, (d) pH 10, (e) pH 11, (f) pH 12.
As shown in Fig. 5, in the case of the treatment without contact with AA5083 (see Fig. 1(c)), no change occurred in AISI 1045 carbon steel at all pH values. In general, steel corrosion is prevented by contact with Al alloys, and it is not surprising that corrosion does not occur on AISI 1045 carbon steel in the conversion treatment under galvanically coupled conditions with AA5083 alloy.
3.3 Galvanic corrosion resistance of AA5083 treated under galvanically coupled conditionThe galvanic corrosion resistance of AA5083 treated under galvanically coupled conditions with AISI 1045 carbon steel was also assessed. As shown in Fig. 4(f), severe corrosion was generated on AA5083 during the conversion treatment at pH 12. Therefore, the galvanic corrosion resistance of the specimens treated at pH ranges of 8–11 was compared. Figure 6 shows the effect of the solution pH in the conversion treatment on the galvanic current densities and electrode potentials of the AA5083 alloy coupled with AISI 1045 carbon steel. As a reference, the results of galvanic coupling of as-polished specimens are presented. In all the cases, large current densities were observed during the initial period. This period of high current density was relatively long for the specimens treated at pH 8 and 9, and short for the specimens treated at pH 10 and 11. As will be discussed later (see Section 3.4), localized corrosion was determined to occur during this period because the electrode potential in this period exceeded the pitting potential of AA5083.

Time variations in (a), (b) galvanic current densities and (c), (d) electrode potentials of conversion-treated AA5083 coupled to conversion-treated AISI 1045 carbon steel in the naturally aerated diluted synthetic seawater at pH 8.2 (298 K). (b), (d) Enlarged views of the initial period of (a) and (c). As the conversion treatment, AA5083 alloy and AISI 1045 carbon steel were immersed in 50 mM Na2MoO4 at pH 8–11 under galvanically coupled condition.
Figures 7(a)–(e) show optical micrographs of galvanic corrosion damages on the AA5083 after galvanic current and potential measurements shown in Fig. 6. The generation of localized corrosion was observed on as-polished and conversion-treated AA5083 specimens. The morphology of the localized corrosion appeared to be pitting corrosion, but close inspection revealed that the corrosion damages were dense filiform corrosion. For simplicity, these localized corrosion damages shown in Figs. 7(a)–(e) are called as pitting corrosion in this study. On the specimen treated at pH 11, small filiform corrosion was generated, as shown in Fig. 7(f). On the as-polished AA5083 and the AA5083 conversion-treated under galvanically coupled condition at pH ranges of 8–11, many pits with a diameter of 300 µm were generated. However, in the case of the AA5083 treated at pH 11, the size of pits was relatively small, and only small filiform corrosion appeared. An area of 10 mm × 10 mm was observed by an optical microscope to count the number of localized corrosion damages, and the results are summarized in Fig. 8. Pitting corrosion and filiform corrosion were counted separately. Comparing the specimens treated at pH ranges of 8–10 and as-polished specimen, there is no significant difference in the total number of localized corrosion damages. On contrary, only two pits and five filiform corrosion damages were observed in the AA5083 treated at pH 11. In Fig. 8, the number of localized corrosion damages on the AA5083 treated under uncoupled condition was significantly low. However, the conversion treatment at pH 11 could provide better corrosion resistance in the specimens conversion-treated under galvanically coupled condition.

Optical micrographs of (a) as-polished and (b)–(f) conversion-treated AA5083 after galvanic current and potential measurements shown in Fig. 6. The conversion treatment was performed in 50 mM Na2MoO4 at (b) pH 8, (c) pH 9, (d) pH 10, (e) and (f) pH 11 under galvanically coupled condition.

Effect of solution pH used in the conversion treatment on the number of localized corrosion damages on as-polished and conversion-treated AA5083 after galvanic current and potential measurements as shown in Fig. 6.
Polarization curves were measured to elucidate the reason why AA5083 treated at pH 11 indicated better galvanic corrosion resistance in naturally aerated diluted synthetic seawater at pH 8.2. Figure 9 shows the potentiodynamic cathodic polarization curves of the as-polished AISI 1045 carbon steel and the carbon steel treated under galvanically coupled conditions. There was no clear difference in the cathodic current densities for all cases. Therefore, the anodic polarization behavior of the AA5083 specimens was supposed to determine their galvanic corrosion resistance in diluted synthetic seawater. Figure 10 shows the potentiodynamic anodic polarization curves of AA5083 treated under galvanically coupled conditions. In this figure, the conversion treatment contributed to improve the pitting corrosion resistance because the pitting potential was increased by the conversion treatment. However, no correlation was observed between the pitting potentials and galvanic corrosion resistance, as shown in Fig. 8. A comparison of Figs. 10 and 6(b) shows that the electrode potential of the AA5083 specimens exceeded the pitting potential in the initial period of galvanic coupling. Therefore, it can be concluded that localized corrosion initiated during the initial period. The current values were also high during this period, indicating that corrosion occurred at the beginning of galvanic coupling. However, the polarization behavior did not reveal why AA5083 treated at pH 11 exhibited excellent galvanic corrosion resistance in diluted synthetic seawater.

Potentiodynamic cathodic polarization curves of as-polished and conversion-treated AISI 1045 carbon steel in the naturally aerated diluted synthetic seawater at pH 8.2 (298 K). As the conversion treatment, AISI 1045 carbon steel and AA5083 alloy were immersed in 50 mM Na2MoO4 at pH 8–11 under galvanically coupled condition.

Potentiodynamic anodic polarization curves of as-polished and conversion-treated AA5083 in the naturally aerated diluted synthetic seawater at pH 8.2 (298 K). As the conversion treatment, AA5083 alloy and AISI 1045 carbon steel were immersed in 50 mM Na2MoO4 at pH 8–11 under galvanically coupled condition.
In the pit initiation of Al alloys, the alkalization around IMPs due to oxygen reduction reaction is recognized as the first step. Afterwards, the alkalization causes the dissolution of the surrounding Al-matrix.44–48) In this study, the effect of the conversion treatment on the cathodic activity of oxygen reduction reaction on IMPs and the alkaline dissolution resistance of the surrounding Al-matrix were analyzed separately. This study focused on Al6(Fe, Mn) particles on AA5083 because filiform corrosion was reported to be initiated at Al6(Fe, Mn) particles in AA5083 under coupled to pure Fe.25) Bulk Al6(Fe, Mn) specimens were fabricated by arc melting to assess cathodic activity, and pure Al was used to evaluate the alkaline dissolution of the Al-matrix on AA5083.
Figure 11 shows the potentiodynamic cathodic polarization curves of conversion-treated bulk Al6(Fe, Mn) in naturally aerated diluted synthetic seawater at pH 8.2. The electrode potential of the bulk Al6(Fe, Mn) specimens was kept at a constant by a potentiostat: −0.30 V at pH 8, −0.30 V at pH 9, −0.40 V at pH 10, and −0.80 V at pH 11, to simulate the conversion treatment under galvanically coupled condition. These setting potentials were determined based on the electrode potential during conversion treatment under galvanically coupled conditions, as shown in Fig. 3(b). In Fig. 11, the cathodic current above −0.9 V was likely to be due to oxygen reduction reaction and decreased with increasing pH. Additionally, the OCP decreased with increasing pH. In Fig. 6(b), the electrode potentials of the AA5083 specimens under galvanic coupling in the diluted synthetic seawater were approximately −0.6 V. Therefore, Al6(Fe, Mn) particles would not be a preferential cathode, and alkalization would suppress when the AA5083 was treated above pH 9. Additionally, no effects of the increase in pH on the surface appearance and polarization behavior were observed on AISI 1045 carbon steel, as shown in Figs. 5 and 10. In this regard, a higher pH would be appropriate for the chemical conversion treatment of AA5083 under galvanically coupled conditions with AISI 1045 carbon steel.

Potentiodynamic cathodic polarization curves of conversion-treated bulk Al6(Fe, Mn) in the naturally aerated diluted synthetic seawater at pH 8.2 (298 K). To simulate the conversion treatment under galvanically coupled condition in 50 mM Na2MoO4, the electrode potential of the bulk Al6(Fe, Mn) specimens was kept at a constant by a potentiostat: −0.30 V at pH 8, −0.30 V at pH 9, −0.40 V at pH 10, and −0.80 V at pH 11.
Figure 12 shows the changes in the OCPs of conversion-treated pure Al in naturally aerated diluted synthetic seawater. As well as the conversion treatment of the bulk Al6(Fe, Mn) specimens, the conversion treatment of pure Al was also performed at a constant potential: −0.30 V at pH 8, −0.30 V at pH 9, −0.40 V at pH 10, and −0.80 V at pH 11 in 50 mM Na2MoO4. The pH of the solution was increased by adding NaOH in a stepwise manner, as shown in Fig. 12. The OCP of the treated Al was initially located around −0.40 V and decreased with time. The OCPs for all specimens decreased and became almost the same when the pH of the solution was increased to 9. The results shown in Fig. 12 suggest that the pH of the solutions used in the conversion treatment did not affect the alkaline dissolution resistance of the Al-matrix on AA5083.

Changes in open-circuit potentials of conversion-treated pure Al in naturally aerated diluted synthetic seawater (298 K). Solution pH was increased by adding NaOH stepwise. To simulate the conversion treatment under galvanically coupled condition in 50 mM Na2MoO4, the electrode potential of the bulk Al6(Fe, Mn) specimens was kept at a constant by a potentiostat: −0.30 V at pH 8, −0.30 V at pH 9, −0.40 V at pH 10, and −0.80 V at pH 11.
The aforementioned results indicate that the decrease in the cathodic activity for oxygen reduction reaction on Al6(Fe, Mn) effectively contributed to improve the galvanic corrosion resistance of AA5083 treated under galvanically coupled with the steel. This can explain why AA5083, treated at the pH 11, exhibited the better galvanic corrosion resistance in dilute synthetic seawater. AES analysis was performed to elucidate the reason for the decrease in the cathodic activity of Al6(Fe, Mn).
Figure 13 shows the AES depth profiles of the Al-matrix on AA5083 treated at pH 8 and 11. The conversion treatment of AA5083 was performed under galvanically coupled conditions using AISI 1045 carbon steel. At pH 8, the thickness of the conversion film was 7 nm, and almost no signal of Mo was detected. In contrast, for the specimen treated at pH 11, the film thickness was 40 nm and approximately 6 at% Mo was detected in the film. However, there was no apparent difference in the alkaline dissolution resistance between the pure Al specimens treated at pH 8 and 11, as shown in Fig. 12. Therefore, this level of film thickening and Mo-enrichment is not expected to be effective in the alkaline dissolution of the Al-matrix on AA5083.

AES depth profiles of the Al-matrix on AA5083 conversion-treated at (a) pH 8 and (b) pH 11. As the conversion treatment, AA5083 and AISI 1045 carbon steel were immersed in 50 mM Na2MoO4 at pH 8 and 11 under galvanically coupled condition.
Figure 14 shows the AES depth profiles of an Al6(Fe, Mn) particle on conversion-treated AA5083 at pH 8 and 11. The conversion treatment of AA5083 was also performed under galvanically coupled with AISI 1045 carbon steel. The thicknesses of the surface films on Al6(Fe, Mn) particles on AA5083 treated at pH 8 and 11 were 4.5 nm and 157 nm, respectively. Additionally, for the specimen treated at pH 8, almost no signal of Mo was detected in the film, but a large amount of Mo was detected on the Al6(Fe, Mn) on AA5083 treated at pH 11. In addition, the Fe concentration decreased at pH 11. The electrical conductivity of Fe-oxides is generally high compared with Al-oxides.49,50) Mo-oxides, such as MoO3, have also been reported to have low electrical conductivity.51) Therefore, the Mo-accumulation in the surface film and the Fe-removal were determined to be the reason for the suppression of cathodic activity for the oxygen reduction reaction on Al6(Fe, Mn), as well as the increase in film thickness.

AES depth profiles of Al6(Fe, Mn) on AA5083 conversion-treated at (a) pH 8 and (b) pH 11. As the conversion treatment, AA5083 and AISI 1045 carbon steel were immersed in 50 mM Na2MoO4 at pH 8 and 11 under galvanically coupled condition.
In general, the surfaces of Al-containing IMPs dissolve in alkali, whereas steels show passivity in alkaline environments. Furthermore, under galvanically coupled conditions, steel corrosion is inhibited, and the anodic dissolution on IMPs on the Al alloy is accelerated. As a chemical conversion treatment of Al alloy/steel joints, conversion treatment with alkali seems to be effective in terms of modifying the surface of IMPs, which act as initiation sites for localized corrosion under galvanic coupling in chloride solutions.
To simulate the chemical conversion treatment of an Al alloy/steel joint, AA5083 alloy and AISI 1045 carbon steel were immersed in 50 mM Na2MoO4 at pH 8–12 under galvanically coupled condition. Afterwards, galvanic corrosion resistance of AA5083 alloy coupled to AISI 1045 carbon steel was assessed in naturally aerated diluted synthetic seawater at 298 K. Moreover, galvanic currents and potentials were measured. Bulk Al6(Fe, Mn) specimens were used to assess the cathodic activity of the intermetallic particles, and pure Al was utilized to evaluate the alkaline dissolution of the Al-matrix of AA5083. The conclusions are as follows.
This work was supported by JSPS KAKENHI Grant Numbers JP21K18804 and JP22H00254. Takumi Kosaba acknowledges support from GP-MS at Tohoku University. The first author (T. Kosaba) was supported by a Grant-in-Aid for JSPS Research Fellow (grant No. JP21J10917). This work was supported by the Light Metal Educational Foundation, Inc. and the aluminium research grant program of the Japan Aluminium Association. A part of this work was carried out at the Research Center for Rare Metal and Green Innovation (RaMGI), Tohoku University, which was established with the support by Ministry of Economy, Trade and Industry (METI) & Ministry of Education, Culture, Sports, Science and Technology (MEXT).