2023 年 64 巻 3 号 p. 627-637
2023 年 64 巻 3 号 p. 627-637
Spent automobile catalysts are the most important secondary resource for platinum group metals (PGMs), and their recycling is essential not only to ensure a steady supply of PGMs but also to preserve the natural environment. However, several factors limit the efficiency of PGM recovery in current recycling processes. Specifically, PGMs represent a small proportion of the entire catalyst and are difficult to dissolve in aqueous solutions for subsequent separation. In recent decades, multiple technologies have been developed to make the recycling of PGMs more efficient and environmentally-friendly. Herein, we introduce the typical industrial processes for recovering PGMs from spent catalysts, along with established and emerging trends in the technological development of PGM recycling. Furthermore, we review novel recycling techniques for converting PGMs in spent catalysts into more soluble states and/or physically concentrating PGMs from spent catalysts.
This Paper was Originally Published in Japanese in J. Japan Inst. Met. Mater. 85 (2021) 294–304.
Platinum group metals (PGMs) consist of 6 elements: Pt, Pd, Rh, Ir, Ru, and Os. In view of their high resistance to heat and corrosion and unique catalytic properties, PGMs find numerous industrial applications. In particular, catalysts for purifying automobile exhaust gas (automobile catalysts) account for a large fraction of the global demand for Pt, Pd, and Rh (Fig. 1).1)
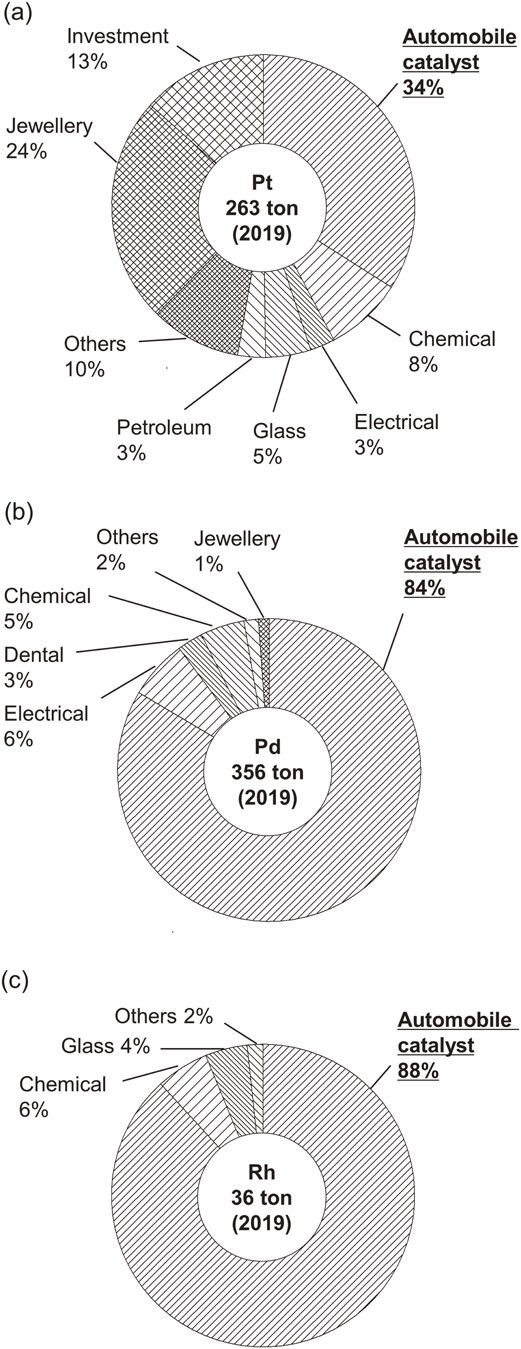
Global demand for (a) Pt, (b) Pd, and (c) Rh in 2019.1)
Over the past 30 years, the demand for PGMs as automobile catalyst components has significantly increased in response to the tightening of environmental regulations and the increasing automobilization of developing countries (Fig. 2).2) Although the shift to electric vehicles will progress mainly in developed countries, the global demand for PGMs as automobile catalyst components is expected to further increase over the next decade or more. Given that the currently recycled catalysts were installed in automobiles manufactured ∼15 years ago, the amount of PGMs recovered from spent automobile catalysts will significantly increase in the coming decades.

Growth in demand of Pt, Pd, and Rh for use in automobile catalysts.2)
Currently, the reserve-to-production ratios do not raise any concern regarding the immediate depletion of PGM natural resources. However, PGM ore deposits are highly localized in South Africa and Russia.3–5) Furthermore, the content of PGMs in the ores is only ∼5 ppm, even in high-grade ores. Thus, millions of tons of ore must be mined and processed to produce just a ton of a given PGM. The production of PGMs from natural resources is highly energy-consuming and results in the discharge of large amounts of gangue and tailings, thereby placing a significant burden on the natural environment around mines and smelters in resource-supplying countries. Therefore, the recovery of PGMs from spent automobile catalysts is important from the perspectives of both resource security and global environment preservation.
Because of the high price of PGMs, they are already being recycled from spent catalysts in industries. However, current recycling processes are highly time- and energy-intensive, consume large amounts of chemicals, and generate huge amount of hazardous waste, which highlights the need for more advanced and efficient recovery technologies.
Japan has a competitive nonferrous smelting industry. Domestic nonferrous smelters not only produce a wide variety of nonferrous metals from imported natural resources, but also collect precious metal (Au, Ag, and PGMs)-containing scraps (end-of-life products and wastes) from around the world and process them for recycling. There are also many companies in Japan that specialize in the recycling and refining of precious metals. With this background, the domestic industry and academia have actively engaged in the research and development (R&D) of the separation and recovery of precious metals. This review outlines the current commercial processes used to recover PGMs from spent catalysts and describes trends in technological development with an aim to upgrade these processes. Furthermore, solubilization techniques and physical concentration processes for PGMs are reviewed as examples of novel technologies developed mainly by domestic researchers.
The appropriate recycling method should be selected according to the content and form of the recovered elements, coexisting substances, and amount of treatment. The industrial recycling of precious metals, including PGMs, is currently accomplished using various methods, because the scale of recycling plants is relatively small, and the economic value of recovered elements is sufficiently high. This section describes the typical methods of recovering PGMs from spent automobile catalysts. Further information on the smelting and recycling of precious metals can be obtained elsewhere.4–14)
Automobile catalysts mostly consist of honeycomb-structured ceramic substrates with a porous catalyst layer (Fig. 3). These substrates are typically composed of cordierite (Mg2Al4Si5O18), which exhibits excellent thermal shock resistance. The catalyst layers are mainly composed of γ-alumina, which has a high specific surface area, and PGMs and their compounds are dispersed and supported as nanoparticles. Additionally, ceria or ceria/zirconia solid solutions are often present in catalyst layers as promoters (or cocatalysts). The composition and amount of PGMs in automobile catalysts vary depending on the type of engine and the production age of the catalyst. A rough estimate of the total content of PGMs in the catalysts is 500–5000 ppm.6) The concentration of PGMs in automobile catalysts is 100–1000 times higher than in natural ores. However, automobile catalysts contain a small amount of PGMs compared to the various PGM-containing recycled raw materials. Furthermore, because of the high chemical stability of PGMs, their separation and recovery from spent catalysts is difficult.
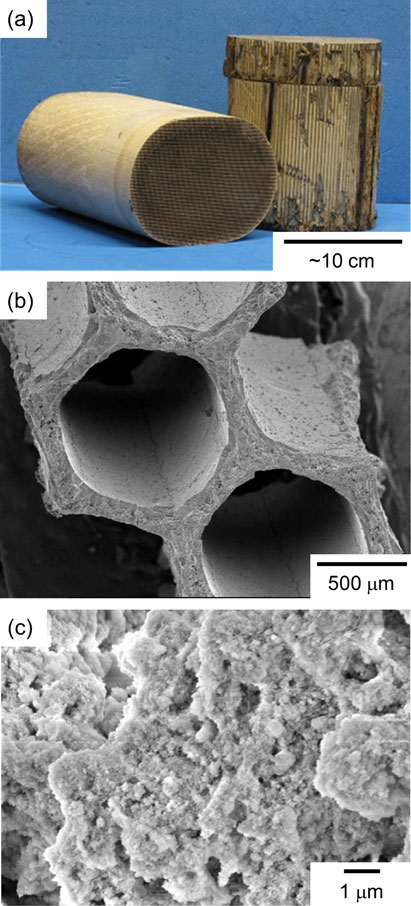
(a) Photograph of automobile catalysts. SEM images of (b) the cross section and (c) the surface of catalyst.
A well-developed network for collecting spent catalysts from end-of-life automobiles allows for the continuous processing of substantial amounts of scrap. Existing smelting techniques developed for natural resource processing (i.e., smelting process of PGMs, Cu, Pb, etc.) can be used to recover PGMs from spent catalysts in high yield. However, locations of the smelter are limited, and the lead time for PGM recovery is long. Because PGMs have a high risk of price fluctuation, it is important to recover PGMs in the shortest possible lead time. Therefore, the recovery of PGMs from spent catalysts is often performed using specialized processes and equipment.
Figure 4 presents an overview of PGM recovery from spent automobile catalysts. Collected catalysts are mechanically pulverized prior to the extraction of PGMs. An accurate assessment of precious metal content in raw materials is important for the recycling business. The homogenization of collected catalysts through pulverization and mixing is an indispensable pretreatment for determining the economic value of the processed scraps.

Typical commercial procedures for recovering PGMs from spent automobile catalysts.
PGM recovery from pulverized spent catalysts is typically achieved through a combination of pyro- and hydrometallurgical processes. The former processes are used to concentrate PGMs, while the latter ones are then used for their final separation and purification. During pyrometallurgical processing, the spent catalysts are smelted with collector metals such as Cu, Fe, and Pb, which results in the extraction of PGMs into the molten metal phase and the removal of cordierite and alumina as slag. Although smelting requires large and specialized equipment, it allows PGMs to be rapidly extracted into alloys in high yields. Moreover, alloying with collector metals facilitates the subsequent dissolution of PGMs in aqueous media.
Smelting processes using Fe as a collector have been conducted in the United States and other countries,15,16) and these processes are based on the heating of pulverized catalysts in a plasma arc furnace together with Fe, fluxes (e.g., CaO), and reductants (e.g., coke). High operating temperatures (1500–1600 °C) are required to maintain Fe and slag in molten states. In Japan, Nippon PGM Co., Ltd. uses a so-called ROSE process, in which the pulverized spent catalysts are smelted in an electric furnace together with Cu or its oxide, fluxes (e.g., CaO and SiO2), and reductants (e.g., coke) (Fig. 5).17–19) The operating temperature (1300–1400 °C) is relatively lower than that employed in the case of Fe. After the removal of slag from the metal phase, the PGM-containing Cu alloy is melted in an oxidizing furnace. Air or oxygen is injected into the melt to gradually oxidize Cu, and the Cu alloy enriched with PGMs is separated and recovered.
In hydrometallurgical (i.e., post-smelting) processing, the PGM-containing alloy is typically dissolved in a strongly oxidizing acidic solution, such as HCl/Cl2 (mixture of hydrochloric acid solution and Cl2 gas) and aqua regia, to efficiently extract PGMs into the solution. After the PGMs are extracted into the acidic solution as chloro-complexes, multi-stage treatment including precipitation, ion exchange, and solvent extraction is conducted to remove non-PGM elements, as well as to separate individual PGMs and recover them as pure metals or compounds. The details of these separation and purification processes are company secrets; however, solvent extraction is often used as the main technology because it enables refining in a shorter time and with a simpler process. The hydrometallurgical processing of PGMs and/or their chemical properties in aqueous solutions have been extensively reviewed.20–25)
PGMs can be directly leached from pulverized spent catalysts without preconcentration by smelting. However, this approach suffers from low recovery of PGMs (especially Rh), the discharge of large quantities of toxic wastewater, and the generation of harmful exhaust gases. Therefore, in countries with strict environmental regulations, such as Japan, direct leaching is currently not implemented on a large industrial scale.
In view of the low content of PGMs in spent catalysts (within several thousand ppm), the difficulty of dissolving PGMs in aqueous media, and the arduousness of separating individual PGMs from their mixtures, the current recycling methods are still time-consuming, labor-intensive, and environmentally hazardous. Consequently, a variety of research has been conducted to achieve more efficient, environmentally friendly, and energy-saving PGM recycling.26–29)
The development of effective dissolution (or leaching) methods for PGMs is one of the most important challenges. To dissolve PGMs in spent catalysts by acid dissolution efficiently, enrichment and alloying by smelting are performed in industries. However, such high-temperature treatment requires the use of large and costly equipment and consumes large amounts of energy, solvents, and reducing agents. Additionally, even after smelting, strong acids with high oxidizing power must be used to dissolve PGMs, and the process is inherently environmentally hazardous in terms of generation of toxic waste liquids and exhaust gases. For this reason, R&D aimed at establishing a dissolution method that directly leaches PGMs from spent catalysts and/or has a low environmental impact has been actively conducted to date. Many studies have focused on the optimization of extraction medium parameters such as solution composition (oxidizing agent, complexing agent, acid concentration, etc.) and temperature. Furthermore, in recent years, pretreatments for changing the chemical state of PGMs (solubilization) are also being actively researched. In Japan, Okabe and Maeda’s group (Institute of Industrial Science, The University of Tokyo) has been conducting pioneering research on this solubilization technology since the early 2000s.30–34) The details of solubilization processes studied by them and other groups are described in Section 4.1.
R&D on the separation and purification (mutual separation) of PGMs using solvent extraction is also very active. The solvent extraction method was introduced to separate and purify PGMs in the 1970s and has developed as an efficient alternative to conventional methods such as precipitation. Currently, solvent extraction is industrially used in the separation of Pt and Pd; however, the employed extractants present the drawbacks of insufficient extraction speed and low stability in acidic solutions. Furthermore, effective solvent extraction techniques for Rh, Ru, and Ir are not well established. Therefore, much effort is currently being directed at the optimization of extraction conditions and the development of more efficient extractants. R&D on this aspect is also active in Japan. For example, Narita’s group (National Institute of Advanced Industrial Science and Technology) reported that amide-containing tertiary amine compounds show excellent performance for Rh extraction from hydrochloric acid solutions.35) Further details on recent research trends in solvent extraction can be found elsewhere.24,36–38)
Other separation and recovery technologies are also being improved and developed from various perspectives. The authors believe that the direct concentration of PGMs using physical separation techniques is particularly beneficial and important. Physical separation techniques are originally developed to crudely separate solid particles in mineral processing, and include particle size classification, gravity separation, flotation, and magnetic separation. Compared to metallurgical processes, physical separation can be performed at a lower cost using simpler equipment. Generally, all of the pulverized spent catalysts are metallurgically processed for extracting PGMs. If physical separation can be incorporated as a pretreatment to concentrate PGMs through the rough removal of non-recoverable ceramics, subsequent extraction can be conducted more efficiently. Thus, physical concentration will be a key pretreatment technology for reducing the energy consumption and environmental impact of PGM recycling.
Development of effective physical concentration technology may result in new material flows and business models for recycling spent catalysts because PGMs can be crudely separated at spent catalyst collection sites and automobile dismantling yards. PGMs are both expensive and fluctuate greatly in price. Thus, shortening of the lead time from spent catalyst collection to refined PGM production is an important issue. Currently, the collection of spent catalysts from used automobiles is conducted worldwide including urban areas, whereas smelters and recycling plants for extracting PGMs from scrap are limited in location because of the need for special equipment. Therefore, the collected spent catalysts are often transported by way of sea or land for further treatment in smelters or recycling plants. The concentration of PGMs at spent catalyst collection sites will improve the economic value of the collected scraps and will reduce the volume of scrap to be transported. With a higher economic value, the processed scrap could then be transported by air, which will significantly reduce the lead time for recovering PGMs from old automobiles.
Owada’s group (Waseda University) has studied the physical separation of PGMs from spent automobile catalysts since the 1990s.39–44) In recent years, Okabe’s group (Institute of Industrial Science, The University of Tokyo) has proposed a new group of physical concentration technologies combining chemical pretreatments,45) some of which have been demonstrated to be effective. Further details of research on physical concentration techniques are described in Section 4.2.
Okabe and Maeda’s group proposed a solubilization process for precious metals existing as trace components in complex structures (e.g., PGMs in spent catalysts); the proposed process involves an alloying treatment using metal vapor, as shown in Fig. 6, and its development has been underway since the early 2000s.10,14,30–34,46–53) Some active metals used, such as Mg, Ca, and Zn, are highly volatile. Their vapors can be easily supplied to the surface of the porous catalyst layer and are suitable as reactants for alloying PGMs. In typical commercial recycling processes, PGMs are extracted and separated from spent catalysts as alloys with collectors, such as Fe and Cu, through pyrometallurgical smelting. However, temperatures required for such conventional techniques are very high (>1200 °C), and a large amount of collector metals must be fed for the process. The alternative treatment with active metal vapor can be performed at significantly lower temperatures (<1000 °C) than smelting, and also minimizes the amount of alloying metal required and consumed.
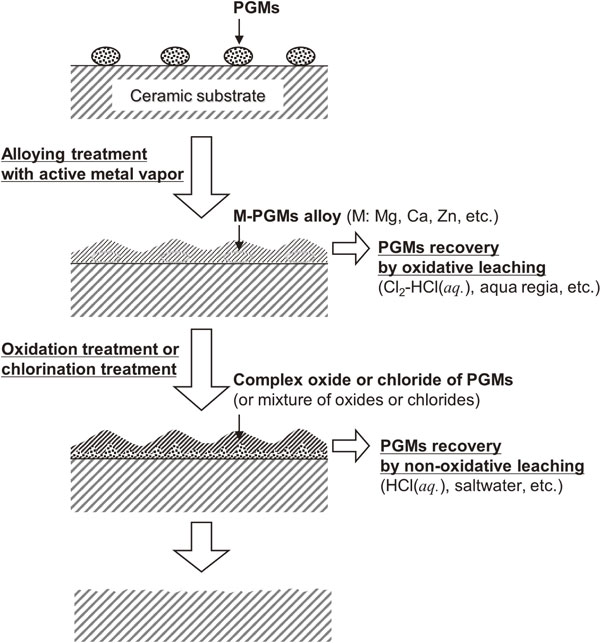
Figure 7 shows the representative results demonstrating the effectiveness of the alloying treatment using active metal vapors.10,46–48,50) Among PGMs, Pt and Rh are particularly difficult to dissolve in acid. Powder samples of Pt and Rh were exposed to the vapors of Mg, Ca, Zn, and Li under a reducing atmosphere at 700–800 °C and subsequently leached with aqua regia. Even under the leaching condition where less than 10% of the untreated sample was dissolved, PGMs were effectively leached after the treatment with metal vapors. The increased dissolution ratio was explained by the preferential dissolution of active metals from PGM-containing alloys, inducing the remaining PGMs to become porous and particulate. Porous and fine particulation increases the reaction area in contact with aqueous solutions. Additionally, through investigation of the anodic dissolution behavior of Zn–PGM alloys in HCl(aq.),54–57) it has been shown that the change in the chemical state of PGMs owing to atomization (i.e., thermodynamic destabilization due to increased surface energy contribution) also contributes to their dissolution.

It was also reported in the early 2000s that oxidation treatment after alloying with active metals enables the dissolution of PGMs in HCl solution without the addition of oxidizing agents. Figure 8 shows the results of leaching tests on Ca–Pt alloy obtained by Ca vapor treatment and on a sample of the same alloy oxidized in air at 900 °C for 24 h.47) For the sample after atmospheric oxidation, Pt could even be extracted using oxidant-free concentrated HCl(aq.), likely because the Ca–Pt alloy was converted into a complex oxide. However, the chemical state (e.g., valence) of Pt after solubilization treatment and the details of the dissolution reaction in HCl(aq.) have not been elucidated.
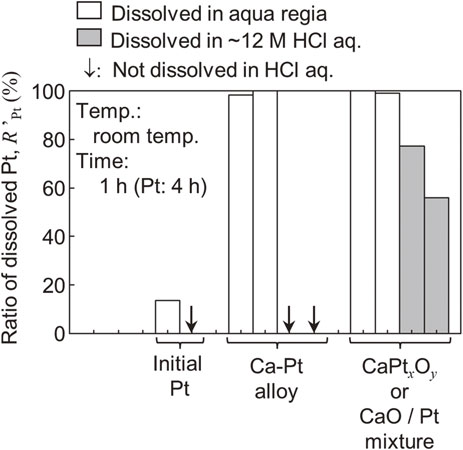
Extraction of Pt from pure Pt metal, Ca–Pt alloy, and oxidized Ca–Pt alloy using aqua regia or concentrated HCl solution at room temperature.47) The Ca–Pt compound, which was obtained via alloying treatment with Ca vapor and subsequent heat treatment at 900 °C in air, was dissolved in HCl solution.
The environmental impact of the dissolution step in recycling can be significantly reduced if leaching of PGMs can be performed with oxidant-free HCl(aq.) rather than with strongly oxidizing acids such as HCl/Cl2 and aqua regia. Recently, Okabe’s group further investigated chemical treatment after alloying with active metals and reported the usefulness of chlorination.51) When a Mg–Pt alloy was mixed with CuCl2 and reacted at 500°C for 3 h, the obtained sample completely dissolved in concentrated HCl(aq.) at 80 °C after 0.25 h (Fig. 9).51,52) When Pt was subjected to chlorination and leaching under the same conditions without prior alloying treatment, its dissolution efficiency was as low as 21%. The chemical state of the Mg–Pt sample after chlorination remains unclear, for example, it is not known whether the product was a complex chloride or a mixture of MgCl2 and PtClx. Nevertheless, alloying with an active metal followed by chlorination has high potential as an effective solubilization technique for PGMs or poorly soluble metals.
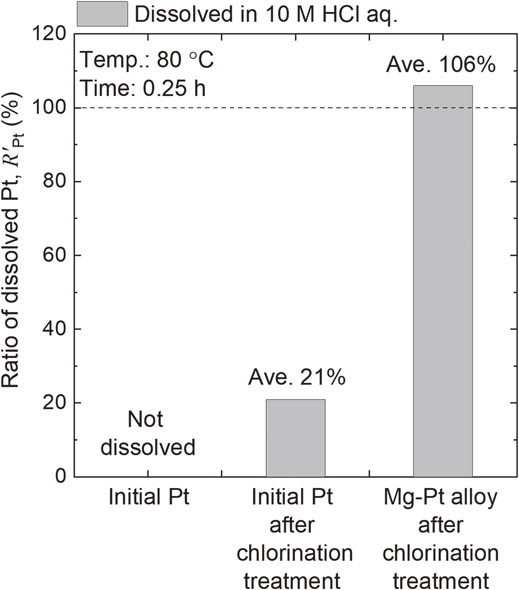
Extraction of Pt from pure Pt metal, chlorinated Pt, and chlorinated Mg–Pt alloy using concentrated HCl solution at 80 °C. The Mg–Pt compound, which was obtained via alloying treatment with Mg vapor and subsequent chlorination treatment using CuCl2 at 500 °C, was dissolved in HCl solution. This figure is reconstructed from Ref. 51) with permission from Springer Nature.
In the experiment shown in Fig. 9, the chlorinating agent (CuCl2) was physically mixed with the Mg–Pt alloy, but in principle, chlorination by gas phase reaction is also possible. For example, Cl2 generated by the thermal decomposition of CuCl2 (CuCl2 → CuCl + 0.5 Cl2) can act as a chlorinating agent. Under appropriate conditions (temperature, chlorinating agent, and reaction vessel shape), the PGM alloy in catalyst scraps can be directly and efficiently chlorinated through a gas-phase reaction. At present, this technology is still in its infancy and has only been successfully demonstrated by Pt powder processing. For practical use of this technology, further studies on the relationship between process conditions and PGM leachability, as well as the reaction behavior of the ceramic components of spent catalysts, are required.
In recent years, R&D on the solubilization pretreatment for PGMs has been actively pursued. In addition to the new technology, which is based on alloying treatment using active metal vapors (Fig. 6), various other processes have been proposed, and the usefulness of some of these processes has been demonstrated.
For example, Nomura’s group (National Institute of Advanced Industrial Science and Technology) reported a process that utilizes oxidative volatilization of PGMs and the absorption of PGM oxides in LaScO3-based perovskite-type oxides.58,59) Although the proposed process requires a high temperature of over 1500 °C, it allows PGMs to be physically separated from scrap via the gas phase and collected as complex oxides that can more easily dissolve in acid. Additionally, Nagai et al. (Chiba Institute of Technology) proposed an advanced version of this method in which Pt, Pd, and Rh are absorbed by different oxides, and they are searching for suitable oxides.60,61)
The oxidation treatment proposed by Kasuya’s group (National Institute of Advanced Industrial Science and Technology) is a solubilization process that has seen significant R&D.62–70) In this method, alkali metal carbonates such as Li2CO3 and Na2CO3 are mixed with pulverized spent catalysts and calcined in air at 600–800 °C. As a result, PGMs are converted into alkali metal–PGM complex oxides (e.g., Pt + Li2CO3 + O2 → Li2PtO3 + CO2), which can be dissolved in concentrated HCl(aq.) without the addition of strong oxidants. Experiments with PGM powders revealed the relationship between the process conditions and reaction products as well as the dissolution behavior of the produced oxides.62–67,69) PGM-loaded alumina powder and pulverized spent catalysts were also processed, and effective extraction of PGMs with HCl(aq.) was demonstrated.68) An important advantage of this new technology is that the solubilization of PGMs in spent catalysts can be achieved by relatively simple and mild pretreatment. Among the investigated alkali metal carbonates, Li2CO3 exhibited the best performance. The alumina in the catalyst also reacts with the alkali metal salt, and in some cases, becomes soluble in HCl(aq.). Therefore, reduction of the required amount of lithium salt and suppression of the solubilization of alumina contained in spent catalysts will be important issues for the practical development. In addition, because the complex oxides are believed to form only when the PGMs and alkali metal salts are in physical contact, the particle size of alkali metal carbonates and their mixing with the spent catalyst will be important factors affecting the process efficiency in practical applications.
The conversion of PGMs to complex chlorides by chlorination in molten salts has also been investigated.71–73) For example, Yoshimura et al. (Chiba University) reported that Pt can be converted to K2PtCl6 by keeping in FeCl3–KCl molten salt at 350 °C.72)
4.2 Physical concentration of PGMs in spent catalystsIn spent catalysts, the concentration of PGMs to be extracted and recovered is less than 1 mass%. Considering the overall recycling efficiency, it is beneficial to use physical separation prior to metallurgical processing to coarsely remove non-recoverable ceramics and concentrate PGMs.
Owada’s group conducted pioneering research on the physical concentration of PGMs in spent catalysts. In the early 1990s, this group processed pulverized spent catalysts with a wet high-intensity magnetic separator, and it was reported that PGMs could be separated when the catalysts contained Ni as a promoter.39,40) However, as only old-type automobile catalysts contain a sufficient amount of Ni, the development of this method has not progressed since then. Owada’s group also studied the enrichment of PGMs by flotation, which is a solid/solid separation technique exploiting differences in surface hydrophilicity.40,41) Solid particles are suspended in an aqueous solution into which bubbles are blown, and particles with a hydrophobic surface attach to the bubbles to float and separate. The usefulness of flotation using dodecylamine hydrochloride or sodium dodecyl sulfate as the collecting agent has been preliminarily investigated, but the method has been limited by the low separation efficiency of PGMs.
Since the 2000s, there have been several reports on physical concentration based on particle size sorting. As the catalyst layer is more fragile than the cordierite substrate, PGMs are preferentially distributed in the finer particles than in the coarse particles after pulverization. Kim et al. (Korea Institute of Geoscience and Mineral Resources) conducted attrition scrabbing under wet conditions and reported that the recovery of Al2O3 and total PGMs in particles with size <300 µm increased with increasing residence time.74) Under appropriate conditions, the recovery efficiency of PGMs reached ∼80%, and the concentration factor reached ∼2.5. A similar study was conducted by Owada’s group wherein it was confirmed that a combination of jaw crusher, roll crusher, and surface grinding using an attritor promotes the distribution of PGMs to fine particles.43) Furthermore, based on mathematical calculation and modeling, Owada’s group reported that heating-quenching of spent catalysts will be a useful pretreatment step for the physical concentration of PGMs.42,44) When spent catalysts are heated to 600–800 °C and subsequently quenched in water, the interface between the catalyst layer and substrate cracks due to differences in their thermal expansion rates. After thermal shock and appropriate pulverization, PGMs are expected to be more efficiently concentrated by particle size sorting.
Although several physical concentration processes have been studied, an effective method that can be widely used in industry has not yet been established. Recently, Okabe’s group has proposed a new technology combining chemical treatment with physical separation (Fig. 10)45) and is working to demonstrate the feasibility and usefulness of this approach.
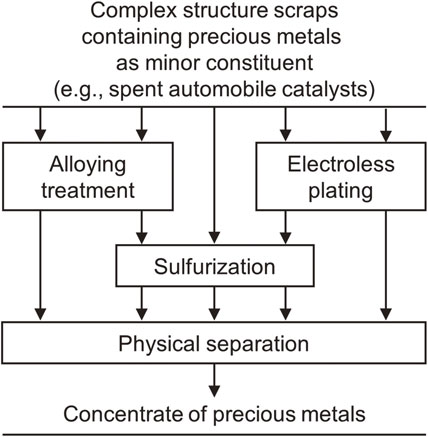
Novel physical concentration techniques in which chemical pretreatments are used to improve the efficiency of PGM separation from scraps (e.g., spent automobile catalysts).45)
For example, the usefulness of a technique combining electroless plating and magnetic separation (Fig. 11) has been demonstrated in a series of studies by the present authors.75–77) In this technique, a ferromagnetic metal (Ni or Fe) is deposited on the surface of PGM particles and/or the porous catalyst layer using electroless plating. To improve the adhesion between the deposited ferromagnetic metal and the catalyst layer and to promote alloying of PGMs with the deposited metals, heat treatment is performed as necessary. Subsequently, the spent catalysts are pulverized, and PGMs are magnetically separated and concentrated.
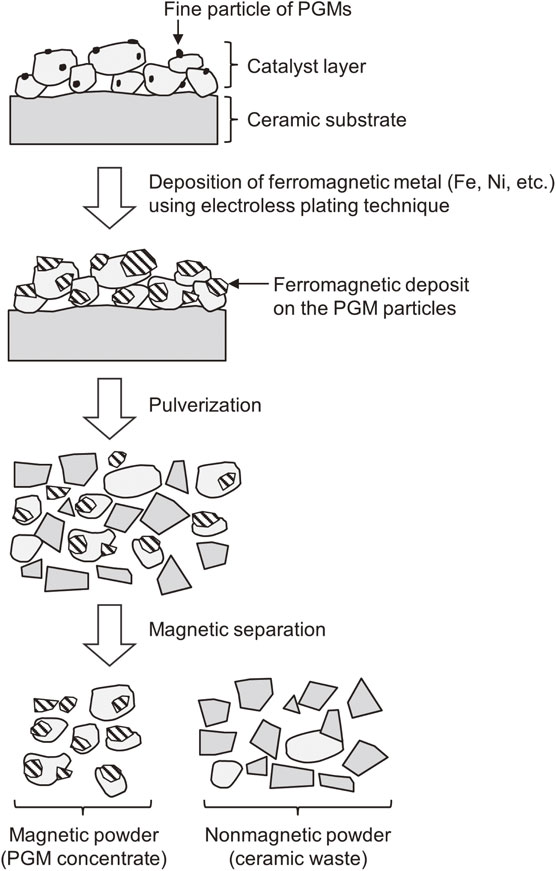
Electroless plating on ceramics usually involves complicated surface pretreatments, such as sensitization and activation. However, when the appropriate plating conditions (such as bath composition and temperature) are selected, PGM particles act as catalyzer and promote electroless plating of Fe and Ni on the catalyst surface without any special pretreatment (Fig. 12). Pt, Pd, and Rh were efficiently concentrated when the simulated catalysts processed by electroless plating were subjected to pulverization and magnetic separation. However, enhancement of process efficiency is essential for the practical use of this technology, as PGM concentration factor and recovery rate from actual automobile catalysts were only ∼2 and 70%, respectively. In our earlier studies, pulverization and magnetic separation were performed in a primitive manner (i.e., pulverization by hand using a mortar and separation using a handheld magnet).75,76) The optimization of these physical operations is expected to further improve the efficiency of PGM enrichment and separation.
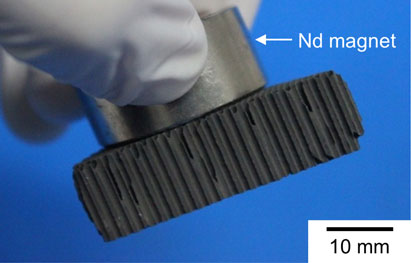
Photograph of a honeycomb-type catalyst sample after electroless Fe plating.77) The attraction of the catalyst sample to the magnet indicates that ferromagnetic Fe was successfully deposited on the surface of the catalyst layer.
The physical concentration process, which combines magnetic separation with a gas-phase chemical reaction for alloying with Fe, is another effective technique for extracting PGMs.77–80) Fe–PGM alloys are ferromagnetic at room temperature when the content of Fe is sufficiently high. Therefore, alloying with Fe is a suitable pretreatment for magnetic separation. However, unlike Mg and Zn, Fe is a low-volatility metal, making it impractical to use Fe metal vapor for the alloying treatment. Consequently, the authors conducted thermodynamic analyses and experiments on alloying treatment using FeClx (x = 2, 3) vapor, showing that PGMs can be efficiently converted to ferromagnetic alloys upon contact with FeCl2 vapor in a reaction system containing metallic Fe.
For example, when Pt powder and a mixture of Fe/FeCl2 powders were vacuum-sealed in a quartz ampoule (Fig. 13(a)) and heated at 927 °C for 1 h to fill the ampoule interior with FeCl2 vapor, Pt was converted to a ferromagnetic Fe–Pt alloy (mainly γ2-FePt with an L10 structure) without physical contact with metallic Fe (Fig. 13(b)).79) Although the disproportionation (3 FeCl2 → Fe + 2 FeCl3) and decomposition (FeCl2 → Fe + Cl2) of FeCl2 vapor can be considered as alloying mechanisms, the former reaction was predicted to be the dominant one through thermodynamic analysis. Other precious metals including Au can also be alloyed with Fe using FeCl2 vapor treatment; in particular, Pd and Rh were converted into ferromagnetic alloys.77,78,80) On the other hand, cordierite and alumina (the major catalyst components) remained unreacted under the same vapor-treatment conditions.80)

(a) Vacuum-sealed quartz ampoule used for the reaction between Pt and FeCl2 vapor. Pure Pt samples were placed in the L-shaped unit and did not come into direct contact with the Fe and FeCl2 powder mixture. (b) Pt powder after alloying treatment with FeCl2 vapor at 927 °C for 1 h. The Pt was converted into ferromagnetic alloy. This figure is reproduced from Ref. 79) with permission from the Mining and Materials Processing Institute of Japan (MMIJ).
Figure 14 shows a schematic of the reaction chamber (that could be industrially viable) and the underlying mechanism for alloying treatment. The spent catalysts are heated and held in a steel chamber filled with FeCl2 vapor. The disproportionation of FeCl2 proceeds on the surface of PGM particles in the spent catalysts, and the PGMs are alloyed with Fe. FeCl3 vapor, a byproduct of the disproportionation reaction, diffuses in the gas phase and reacts with metallic Fe (e.g., inner wall of steel chamber) to regenerate FeCl2. Overall, FeCl2 vapor serves as a medium for transporting Fe into PGMs and is not consumed in principle. As spent catalysts have a complex and porous surface structure, FeClx species may attach to their surfaces in large quantity after the alloying treatment. However, these species can be easily separated and recovered from catalyst scraps via volatilization (e.g., vacuum distillation). Therefore, the consumption of FeCl2 and the generation of toxic waste can be minimized.

In fundamental experiments with simplified catalyst samples, PGMs were successfully concentrated by magnetic separation after alloying treatment with FeCl2 vapor and pulverization.80) However, future studies are required to demonstrate the usefulness of this technology using actual spent catalysts as well as to optimize the process conditions.
Chemical treatment with electroless plating and FeCl2 vapor is not only useful as pretreatment for physical separation but also enhances the dissolution of PGMs in acids. For example, when Rh-loaded alumina powder was kept in aqua regia at 40 °C for 1 h, almost no Rh was leached. Meanwhile, after electroless iron plating and heat treatment, almost all of the Rh could be extracted in only 0.5 h due to the alloying effect.81) It is also of interest to note that, after alloying treatment with FeCl2 vapor, Pt powder easily dissolved even in diluted aqua regia.77) Acid dissolution of PGMs in the alloyed state requires an oxidizing agent. However, if chlorination (or oxidation) treatment described in the previous section is applied after the physical concentration, PGMs may be efficiently extracted using oxidant-free HCl(aq.).
Japanese industry and academia have significantly contributed to the R&D of technologies for recovering PGMs from end-of-life products and waste, as exemplified by the solubilization and physical concentration processes introduced in this review. The new technologies presented herein are currently in the basic research stage but have the potential to reduce the environmental impact of PGM extraction, shorten the lead time of PGM recycling, and/or perform the enrichment of PGMs at spent catalyst collection sites using small-scale and simple equipment. A better circulation of PGM resources can be realized if these technologies are further developed and put to practical use.
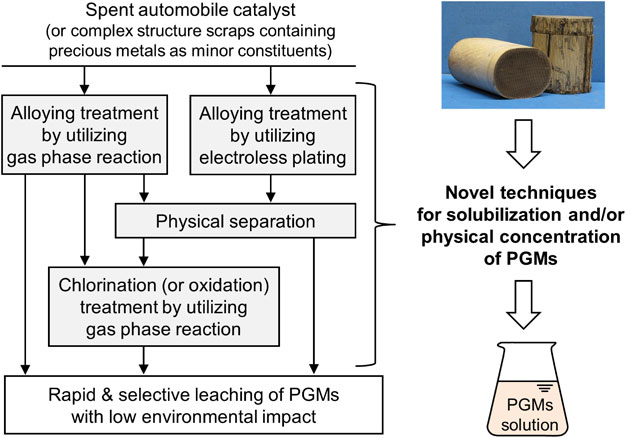
Figure 15 summarizes the solubilization and/or physical concentration methods studied at the Institute of Industrial Science, The University of Tokyo. The ultimate goal of this research is to realize an environmentally friendly process in which PGMs are rapidly and selectively extracted with saltwater without the generation of hazardous liquid waste. Apart from developing such pretreatment technologies, a new process for recycling precious metals using dissolution in molten salt and electrochemical anodic deposition is also studied in the institute and is detailed elsewhere.82)
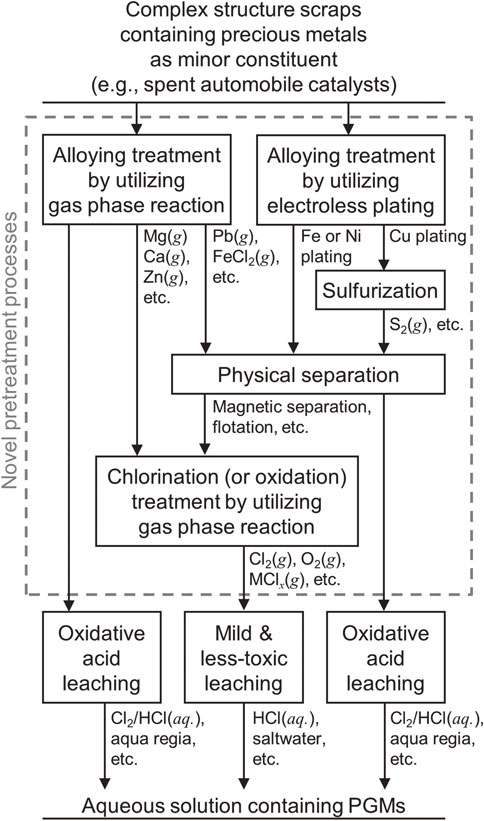
Novel pretreatment processes involving solubilization and/or physical concentration treatment for precious metals in scraps with complex structures (e.g., PGMs in spent automobile catalysts). The pretreatment processes allow precious metals to be leached rapidly and selectively, thereby reducing the environmental burden.
Research on physical concentration processes conducted by the authors (described in Section 4.2) was financially supported by the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Scientific Research (S) (KAKENHI Grant No. 26220910) and by the Mazda Foundation.