2023 年 64 巻 3 号 p. 638-642
2023 年 64 巻 3 号 p. 638-642
For Mg-doped Zn2P2O7, this systematic investigation of co-doping onto Zn sites has elucidated specific effects on negative thermal expansion (NTE). The low-cost and low-environmental-impact NTE material Zn2−xMgxP2O7 shows large NTE in a temperature range including room temperature for x = 0.4. Although Mg doping broadens the operating-temperature window, it remains several dozen degrees wide. Moreover, the total volume change related to NTE becomes less than that of the Zn2P2O7 parent material. Findings obtained from this study demonstrate that co-doping of Mg and of another element onto the Zn site is effective for achieving simultaneous expansion of the operating-temperature window and maintenance of the volume change related to NTE. One illustrative case is that Zn1.64Mg0.30Al0.06P2O7 has a large negative coefficient of linear thermal expansion of about −65 ppm/K at temperatures of 300–375 K. In fact, at temperatures high above room temperature, Zn1.64Mg0.30Al0.06P2O7 powder shows better thermal expansion compensation capability than the composition without Al. The Al-doped phosphates are expected to have broad practical application because of their performance, cost, and environmental load characteristics.
Co-doping of Mg and of Al onto the Zn site is effective for achieving simultaneous expansion of the operating-temperature window and maintenance of the volume change related to negative thermal expansion.
Control of thermal expansion is necessary because even slight thermal expansion can strongly and adversely affect device performance. One promising approach to control thermal expansion uses negative thermal expansion (NTE) materials1–3) which, unlike most usual materials, contract when heated. This approach is anticipated for application in various fields including high-precision optical instruments and electronic devices. Recently, pyro-vanadates and phosphates A2B2O7 (A: alkaline metals, transitional metals; B: V, P)4–7) have attracted great attention as promising NTE materials. Particularly, Zn2P2O7 exhibits a first-order phase transition from the low-temperature (low-T) α-phase to the high-T β-phase at 405 K, which accompanies contraction of its unit-cell volume by 1.8%.8–10) By substituting Zn with Mg, the abrupt volume change became gradual. The operating-temperature window was expanded. In fact, Zn1.6Mg0.4P2O7 exhibits a large negative coefficient of linear thermal expansion αL exceeding −60 ppm/K at 280–350 K (ΔT = 70 K).11) It is anticipated for practical use because it contains no toxic or expensive elements. In fact, it can be fabricated using simple solid state reaction methods.
However, Mg doping reduces the total volume change ΔV/V of 1.8% exhibited by Zn2P2O7 to about 1.4%. The gradual change in volume with respect to T is insufficient for widespread practical use. Results of our preliminary studies suggest that co-doping a third Zn-site element in addition to Mg is effective for improving these functionalities.11) For example, a few percent Cu substitution expands the operating-T window dramatically. However, the total volume change is reduced simultaneously. A strategy for improving the NTE properties of Zn2−xMgxP2O7 is strongly desired.
We have systematically investigated how the crystal structure and thermal expansion properties of Zn2P2O7 vary according to doping of the third Zn-site element in addition to Mg. Examination of several elements including Cu, Fe, Mn, and Al as the third element revealed that a small amount of Al or Mn simultaneously increased the total volume change ΔV/V and the width of the operating-temperature window ΔT. Optimization of the third element can improve the NTE performance while retaining material benefits such as low cost and low environmental impact. Particularly, the optimization facilitates excellent thermal expansion control, especially in the high-T region.
Polycrystalline samples of Zn2−x−yMgxAyP2O7 (A = Al, Cu, Fe, Mn) were prepared using solid state reactions.11) Starting materials for the present experiments were powders of ZnO (99.9% purity), MgO (99.99% purity), α-Al2O3 (99.9% purity), CuO (99.99% purity), α-Fe2O3 (99.9% purity), MnO (99.9% purity), and (NH4)H2PO4 (99% purity). They were weighed at appropriate molar ratios, mixed, and calcined at 800°C in air for 2 h. The obtained powder was crushed, pressed into a pellet, and then sintered at 875°C in air for 2 h. Linear thermal expansion ΔL(T)/L was measured at 100–500 K using a laser-interference dilatometer (LIX-2; Ulvac PHI). X-ray diffraction (XRD) experiments were conducted at 110–400 K at the Aichi Synchrotron Radiation Center, Japan, with synchrotron radiation of λ = 0.62032 Å. To verify the thermal expansion compensating capability, powder obtained by grinding of the sintered materials was composited with epoxy resin using a rotational molding method.12) The epoxy resin matrix was a two-component epoxy resin adhesive prepared by mixing of the resin (EP-4100E; Adeka Corp.) and hardener (EH-105L; Adeka Corp.) in a mass ratio of 100:30. The respective specific gravities of the resin and hardener were 1.17 and 1.13 g/cm3. The linear thermal expansions ΔL/L of the composites were measured using a strain gage (KFL; Kyowa Electronic Instruments Co., Ltd.) with a Cu reference at T = 100–400 K.13) Porosity p was estimated using Archimedes’ method (SD-200L; Alfa Mirage Co., Ltd.).
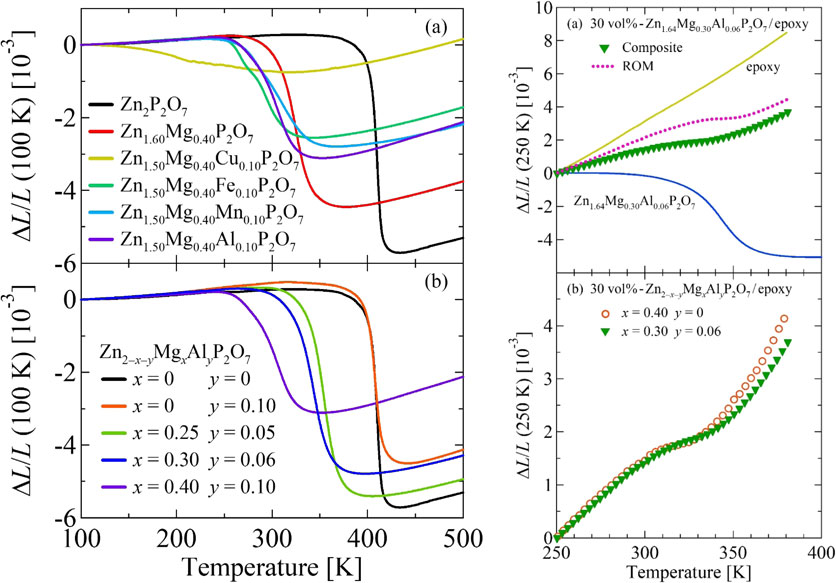
Figure 1(a) presents the linear thermal expansion of Zn2−x−yMgxAyP2O7 (A = Al, Cu, Fe, Mn) sintered body measured using a dilatometer. Dilatometry measurements using sintered samples elucidate the T variation of the bulk volume V as ΔV/V = 3ΔL/L because the crystallographic anisotropy is averaged. Results of an earlier study11) indicated that the transition temperature decreases and that the width of the operating-temperature window increases as the amount of Mg substitution in Zn2P2O7 increases. The total volume change also decreases. The present study revealed that a few percent Al, Fe, or Mn substitution as the third Zn-site element, in addition to Mg, broadens the volume change. Furthermore, the decrease in volume change is suppressed by Al or Mn substitution compared to Cu substitution.

Linear thermal expansion ΔL/L of Zn2−x−yMgxAyP2O7 (A: Al, Cu, Fe, and Mn) (a) and Zn2−x−yMgxAlyP2O7 (b). Data normalized at 100 K. Data were collected using a laser-interference dilatometer during warming.
However, if the Zn content is reduced excessively by co-doping of Mg and the third element, then the volume change is also reduced. Therefore, the strategy was to reduce the Mg substitution amount and thereby stave off the decrease in all volume change, simultaneously compensating with a small amount of the third element to promote the gradual change of volume with T. An illustrative example is the case of adopting Al as the third element described hereinafter. Figure 1(b) represents the linear thermal expansion of sintered specimens for several compositions. The obtained results reveal that the sole substitution of Al at the Zn site does not widen the operating-temperature window of NTE; the substitution of Al is meaningful only when substituted simultaneously with Mg as the third element. For the case of Zn1.70Mg0.25Al0.05P2O7, the operating-temperature window was expanded while maintaining the volumetric shrinkage of pure Zn2P2O7. For another case of Zn1.64Mg0.30Al0.06P2O7, this approach reduced the decrease in the total volume change ΔV/V and maintained it at 1.52%. It also extended the operating-temperature window to 300–375 K. Consequently, the larger NTE of αL = −65 ppm/K was achieved over a wider T-range than for Zn1.6Mg0.4P2O7.
To examine the temperature dependence of the crystal structure and its correspondence to the thermal expansion properties presented in Fig. 1, x-ray diffraction experiments using synchrotron radiation and Rietveld analysis using RIETAN-FP14) (Fig. 2) were conducted for Zn1.64Mg0.30Al0.06P2O7. At 275–375 K, a two-phase analysis assuming the coexistence of monoclinic I2/c (low-T α-phase)9) and monoclinic C2/m (high-T β-phase)8) was conducted with sufficiently high accuracy. The two-phase coexistence indicates that this NTE originates from the volume change associated with the first-order phase transition, as discussed later. Although we attempted similar two-phase analysis at other temperatures, the single-phase analysis was found to be more reliable. Therefore, low-T α-phase was assumed at temperatures below 275 K. The high-T β-phase was assumed at temperatures above 375 K. A very small amount of impurity was suggested by weak peaks (▼: unknown), but the impurity was judged to have no influence on the physical properties or structural analysis discussed below.
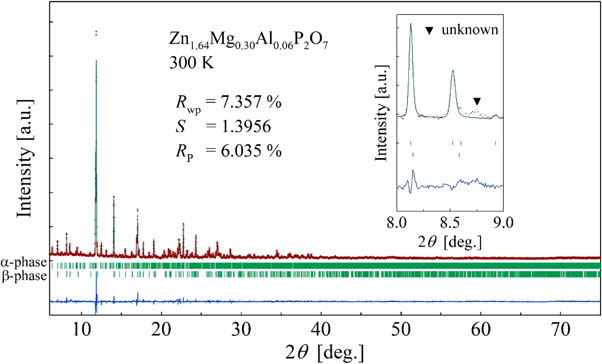
Rietveld refinement of Zn1.64Mg0.30Al0.06P2O7. The experimentally obtained (cross) and fitted (line) patterns are shown. The patterns were measured at 300 K using synchrotron radiation of λ = 0.62032 Å. Vertical bars under the diffraction peaks show the Bragg peak reflection positions. Plots under the bars represent residues. The inset presents a magnified view of the angular region including a weak peak attributable to impurities.
Figure 3 presents the temperature dependence of the lattice parameter of Zn1.64Mg0.30Al0.06P2O7 obtained from the present refinement. In Zn2P2O7, the low-T α-phase takes I2/c; the high-T β-phase takes C2/m. For this phase transition, the a-axis length, b-axis length, and c-axis length of the low-T phase are defined respectively as three times, equal to, and two times larger than those of the high-T phase. Therefore, to compare parameters before and after the phase transition, the low T side was set as a/3, c/2, and v/6. Here, the unit-cell volume v is expressed as $v = abc\sqrt{1 - \cos^{2}\beta } $.

Temperature dependence of crystallographic parameters calculated from Rietveld analysis results at 110–400 K. Circles and diamonds respectively represent the parameters of the low-T α-phase and the high-T β-phase. Open symbols represent values obtained from linear extrapolations of data for other temperatures represented by solid symbols.
The structural change from the low-T α-phase (I2/c) to the high-T β-phase (C2/m) and the temperature dependence of each crystallographic parameter of Zn1.64Mg0.30Al0.06P2O7 are similar to those of Zn2P2O78,9) and Zn1.6Mg0.4P2O7.11) Zn1.64Mg0.30Al0.06P2O7 crystallizes in monoclinic I2/c at 110–275 K, whereas it crystallizes in monoclinic C2/m at 375–400 K. In the 275–375 K transition region, the lattice parameters exhibit sharp changes: the b-axis increased concomitantly with increasing T, whereas the a-axis and c-axis and β decreased, leading to contraction of the unit-cell volume v. As shown in the lower inset of Fig. 4, the fractions of the low-T and high-T phases changed continuously with increasing T. This gradual two-phase switching with respect to temperature apparently causes a gradual volume change with regard to T in the NTE region. Similar gradual volume change with T is also observed for other NTE materials such as Bi0.95La0.05NiO3.15,16) For the T range in which the two phases coexist (275–375 K), weak peaks originated from the phase with the lower fraction. This characteristic prevented us from refining crystallographic parameters such as the lattice constants of both phases with sufficient precision. Refinement was prevented because the three parameters of scale factor, temperature factor, and atomic coordinates are all related to peak intensity. Moreover, they are strongly mutually correlated. However, the temperature range for two-phase coexistence is about 100 K at most. Presumably, the temperature factor and the atomic coordinate change are small in this narrow T-range. Therefore, the lattice parameters were determined in advance to avoid large deviations in peak positions. Only the scale factor was refined; the temperature factor and atomic coordinate parameters were fixed. Because the lattice parameter varies linearly with T in the low-T α-phase and the high-T β-phase, we inferred that this temperature dependence is maintained in the two-phase coexistence region. The fractions of phases determined in this way are shown as open symbols in Fig. 3.
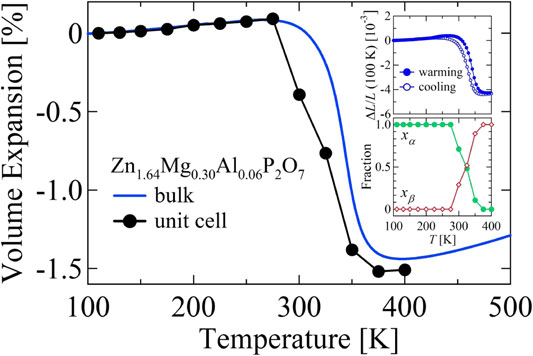
Volumetric thermal expansion of Zn1.64Mg0.30Al0.06P2O7. Data are normalized at 110 K. Results of dilatometry measurements, 3ΔL/L, are compared with the temperature changes of the crystallographic unit-cell volume Δv/v. The upper inset shows the hysteresis loop observed in dilatometric measurements in warming (solid circles) and cooling (open circles) processes. The lower inset shows fractions of the α and β phases: xα and xβ.
Zn1.64Mg0.30Al0.06P2O7 shows anisotropic thermal expansion with b-axis expansion, and with the a-axis and c-axis contracting with increasing T. Anisotropic thermal deformation of the unit cell might produce microstructural effects. In sintered bodies of Cu2V2O74) and its analogues,5) β-eucryptite,17) and Ca2RuO4,18,19) the dilatometric NTE exceeds the crystallographic NTE because of these effects. For Ti2O3,20,21) despite the positive crystallographic thermal expansion, these effects can render the dilatometric thermal expansion negative. Therefore, we examined the microstructural effects in this material as well, which revealed that these effects were less pronounced. Figure 4 shows the bulk volume thermal expansion ΔV/V = 3ΔL/L (blue line) and the crystallographic volume thermal expansion Δv/v (black circles and line). For this study, ΔV/V was estimated using dilatometry for both warming and cooling processes, respectively shown by solid and open symbols in the upper inset of Fig. 4. Also, Δv/v is defined as the fraction-weighted sum of the unit-cell volumes of the two phases. The dilatometric expansion data show hysteresis of about 15 K, at most. In Zn1.64Mg0.30Al0.06P2O7, the unit-cell volume v contracts by 1.61% at 275–375 K, which is not significantly different from the contraction of 1.52% found for the bulk sintered material. This result confirms that the NTE of this material originates from contraction of the unit-cell volume. This finding provides important benefits for engineering because it avoids the following two difficulties associated with microstructural effects. First, microstructural effects can produce microcracks during the thermal cycling process, which might degrade the reproducibility of NTE properties.17) Second, in terms of fine particles,3,22) which are important for thermal expansion control in the local region,23) NTE derived from microstructural effects can be degraded when the microstructure is destroyed by milling.24)
We evaluated the thermal expansion of 30 vol%-Zn1.64Mg0.30Al0.06P2O7/epoxy composites and verified the thermal expansion compensating capability of Zn1.64Mg0.30Al0.06P2O7. The volume fraction was ascertained from the material weights and specific gravities, which are, respectively, 1.16 and 3.20 for the epoxy resin and Zn1.64Mg0.30Al0.06P2O7. Here, we estimated the porosity p of Zn1.64Mg0.30Al0.06P2O7 as p = 0.2 from measurements taken using Archimedes’ method11) and assumed p = 0 for the epoxy resin. Figure 5(a) presents results of ΔL/L measured on the composite. For comparison, data of the pure epoxy resin and the pure Zn1.64Mg0.30Al0.06P2O7 sintered body are also shown. Furthermore, a prediction (rule of mixture, ROM11,12)) based on the volume-weighted sum of contributions from epoxy resin matrix (70 vol%) and Zn1.64Mg0.30Al0.06P2O7 filler (30 vol%) is shown for evaluating the thermal expansion of composites. Results indicate that thermal expansion of the composite was significantly lower than the ROM prediction. In composites of NTE fillers and matrices, the thermal expansion of materials with a larger elastic modulus is known to be reflected strongly because of elastic interaction at the interface.12,25) The results obtained from the present study are attributable to the greater stiffness of Zn1.64Mg0.30Al0.06P2O7 than that of the resin. Figure 5(b) presents a comparison with the thermal expansion of 30 vol%-Zn1.6Mg0.4P2O7/epoxy composites.11) Below 340 K, both show similar thermal expansion characteristics. However, at temperatures higher than 340 K, the thermal expansion of the resin containing Zn1.64Mg0.30Al0.06P2O7 filler lessens because the thermal expansion compensating capability of Zn1.64Mg0.30Al0.06P2O7 filler exceeds that of Zn1.6Mg0.4P2O7 in this higher-T region. This excess originates from the greater total volume change than that of Zn1.6Mg0.4P2O7. Results show that, in the overall T range of 250–380 K, the thermal expansion of the composite with Zn1.64Mg0.30Al0.06P2O7 is lower by about 500 ppm than that of the Al-free counterpart.
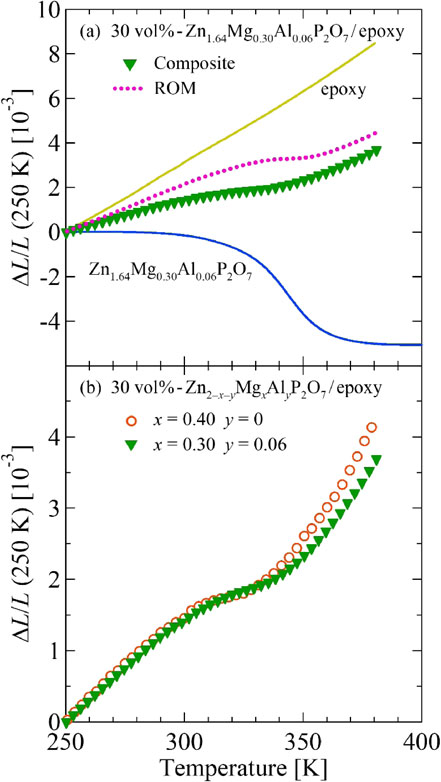
(a) Linear thermal expansion ΔL/L of 30 vol%-Zn1.64Mg0.30Al0.06P2O7/epoxy resin composite (green solid triangles). The dashed line shows volume-weighted sum of contributions from Zn1.64Mg0.30Al0.06P2O7 (30 vol%) and epoxy resin (70 vol%). (b) Comparison with linear thermal expansion ΔL/L of 30 vol%-Zn1.6Mg0.4P2O7/epoxy resin composite (orange open circles). In both plots, the reference temperature is 250 K.
Results obtained from the present study demonstrate that simultaneous doping of Mg and a third element onto the Zn site is effective for tuning the NTE properties of Zn2P2O7. Particularly, the co-doping of Al and Mg increased both the total volume change and the operating-temperature window related to NTE. The reason for this improvement has yet to be explored, but it might be related to a vacancy or excess of atoms attributable to differences in valence. The substitution of trivalent Al for divalent Zn is likely to cause a vacancy in metal ions (Zn2+, Mg2+) and/or an excess of oxygen because of the requirement for electroneutrality conditions. Such a defect might make the volume change associated with the phase transition more gradual. In addition, compared to Zn2P2O7, Mg2P2O7 has a lower structural phase transition temperature of 341 K and a smaller total volume change associated with the phase transition.26) Therefore, reduction of the Mg amount might contribute to suppression of the decrease in the total volume change and might increase the transition temperature (operating temperature). More detailed structural analyses, including those of internal structures such as occupancy and atomic coordinates, will be necessary to clarify them. Manganese is also promising as a third element, partly because the high-T C2/m structure is stable even at a lower temperature in Mn2P2O7 than in Zn2P2O7.8) Furthermore, similarly to Cu, an effect of d electrons might derive from Mn.11) Resolution of this important and interesting issue will require further research.
Even a slight amount of the third element can affect the NTE characteristics considerably. For the case of Zn1.64Mg0.30Al0.06P2O7, the thermal expansion compensating capability at temperatures higher than room temperature was improved. The present material is useful to control the thermal expansion of metals27–29) and engineering plastics30) such as polyimide and polyamide-imide, which are expected to be useful at temperatures higher than those which can be withstood by epoxy resins. This capability represents a great practical merit of the Al-doped pyrophosphates because they are effective at controlling the thermal expansion of devices that generate heat, such as optical and electronic devices.
The NTE properties of Zn2−xMgxP2O7 can be tuned sensitively by doping of a third element onto the Zn site. Optimization of the third element can improve the NTE properties while maintaining its practical benefits of low cost and low environmental impact. One illustrative result, an Al-substituted material, exhibited a greater total volume change and a wider operating-temperature window related to NTE than those of Zn2−xMgxP2O7. For Zn1.64Mg0.30Al0.06P2O7, effective thermal expansion compensation was demonstrated especially at temperatures high above room temperature. The important benefits of high performance, low cost, and low environmental impact of this material are anticipated for widely various engineering applications in the future.
The authors are grateful to K. Morino of ADEKA Corporation for providing epoxy resin. This work was supported financially by Grants-in-Aid for Scientific Research (Nos. JP19H05625 and JP20H00346) from MEXT, Japan, and by SCORE from JST, Japan. Synchrotron radiation experiments were conducted at BL5S2 and the Nagoya University beamline BL2S1 of the Aichi Synchrotron Radiation Center, Aichi Science and Technology Foundation, Aichi, Japan (Proposal Nos. 202104111, 2021L3002, 2021L2002, 2021L1002, and 2020N6004).