2021 年 1 巻 1 号 p. reg-20-reg-25
2021 年 1 巻 1 号 p. reg-20-reg-25
Selenophosphate is an important intermediate for selenoprotein synthesis. Despite its biological significance, the existence of selenophosphate in in vivo samples has not been corroborated by instrumental analyses, such as NMR spectroscopy and mass spectrometry. In this study, we synthesized selenophosphate and subjected it to detailed structural analyses, including 31P NMR, 77Se NMR, and LC-ICP-MS/MS analyses. We confirmed that the structural information of the chemically synthesized selenophosphate was consistent with that of the product biologically synthesized by SelD, a bacterial selenophosphate synthetase. Based on these results, the structure of the SelD product was confirmed.
Selenium (Se) is an element belonging to the same group on the periodic table as oxygen, sulfur, and tellurium, i.e., group 16, and has biologically ambivalent characteristics. Se is an essential element in animals but can be highly toxic when the amount ingested exceeds the nutritional level. Se is required as the active center of such proteins as glutathione peroxidase (GPx), thioredoxin reductase, thyroid hormone deiodinase, and selenoprotein P (Sel P) [1]. These proteins, called selenoproteins, not only function as an anti-oxidant but also participate in thyroid hormone production, DNA synthesis, and fertilization [2– 5]. The active center of selenoproteins consists of a selenol group (-SeH) on a selenocysteine (SeCys) residue in a selenoprotein sequence [1, 6]. Thus, animals have very unique translational machinery for SeCys, which is called the “21st amino acid” [7, 8].
A unique mechanism for selenoprotein synthesis is known [7, 9]. Exogenous SeCys, being a free amino acid, is not incorporated into selenoproteins and hence, SeCys is de novo synthesized on tRNA by the reaction of activated Se, selenophosphate, and activated serine bound to tRNA, and SeCys-binding tRNA (SeCys-tRNASeCys) carries SeCys to the SeCys translation complex on mRNA [10]. Then, SeCys is incorporated into a protein by the UGA codon, one of the most common stop codons [11, 12]. It is known that ingested Se is utilized for selenoprotein synthesis after it is activated to selenophosphate [13]. Therefore, selenophosphate is an important intermediate for selenoprotein synthesis [14, 15].
Selenophosphate is an enigmatic compound. Although the biological significance of selenophosphate has been clearly established [13, 16], its existence in in vivo samples has not been corroborated by instrumental analyses, such as NMR spectroscopy and mass spectrometry. Selenophosphate is highly reactive and sensitive to oxidation. In fact, the half-life of selenophosphate at 0 ºC in air is 32 hr [17]. Stadtman’s group obtained the 31P NMR spectrum of selenophosphate produced by bacterial selenophosphate synthetase, a product of selD [18– 20]. However, their data indicated that the SelD product contained phosphorus (P) having a unique chemical shift (δ = 23.4), differing from the chemical shifts of P in ATP, ADP, and AMP. In other words, there is no evidence that the selD product contains Se and P at the molar ratio 1:1 in its molecule. To confirm the structure of the selD product, the simultaneous detection of Se and P in the molecule is mandatory.
Although inductively coupled plasma mass spectrometry (ICP-MS) is the most sensitive and robust technique for Se detection to date [21], it is less sensitive to P than Se due to the low ionization efficiency of P and the large interference. Thus, it is difficult to detect P at the same detection level as Se. Recently, an inductively coupled plasma tandem mass spectrometer (ICP-MS/MS) has been developed and is commercially available. As ICP-MS/MS can effectively remove the interference and has a unique detection mode using oxygen, it is able to simultaneously detect Se and P.
NMR measurement is also applicable to the simultaneous detection of Se and P because both elements have NMR-active isotopes, i.e., 77Se and 31P (natural abundance is 7.6% and 100%, respectively) [22]. In a previous work, the structure of the SelD product was presumed by 31P NMR measurement [19]. However, the simultaneous detection of Se and P by 77Se NMR measurement would be more reliable for the identification of the structure of the SelD product than the 31P NMR measurement, namely, if a direct magnetic coupling of 77Se nuclei with 31P nuclei is observed, the coupling constant calculated by NMR would provide solid evidence of the existence of a Se=P double bond in its molecule [23]. In this study, we chemically synthesized selenophosphate and identified it by 31P NMR, 77Se NMR, and ESI-MS measurements, in comparison with the reported data of the enzymatic product by SelD. Then, we measured the molar ratio of P and Se in the chemically synthesized compound identified as an equivalent to the SelD product by simultaneous detection using LC-ICP-MS/MS.
Chemicals
Se (metal, powder), tris(trimethylsilyl)phosphite, sodium hydroxide, dithiothreitol, and dimethyl sulfoxide were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other reagents used in this study were of the highest or analytical grade.
NMR analysis
31P and 77Se NMR spectra were obtained by JEOL JNM ECP400 (9.4 T) or JEOL JNM ECP600 (14.1 T) with 1H decoupled. The Larmor frequencies for 31P nuclei were 162 MHz (9.4 T) and 243 MHz (14.1 T), and those for 77Se nuclei were 76.3 MHz (9.4 T) and 114 MHz (14.1 T).
Synthesis of O,O,O-tris(trimethylsilyl)selenophosphate 2 .
Elemental Se (powder, 0.66 g) was suspended in liquid tris(trimethylsilyl)phosphite 1 (2.4 g) under nitrogen atmosphere, and the suspension was stirred at 50 °C for 12 hr. The reaction mixture was filtered to afford 3.0 g (quantitative yield) of O,O,O-tris(trimethylsilyl)selenophosphate. The chemical purity of the product was confirmed by 31P NMR, 77Se NMR, and ESI-MS analyses. 31P NMR (162 MHz, CDCl3) δ 22.3 (s, relative intensity 0.92), δ 22.3 (d, relative intensity 0.08, J31P-77Se = 926 Hz). 77Se NMR (76.3 MHz, CDCl3) δ −158.9 (d, J77Se-31P = 926 Hz). HRMS (ESI) calcd. for C9H28O3PSeSi3+ [M+H]+: 379.0244, found: 379.0248.
Synthesis of trisodium selenophosphate
An aqueous solution of NaOH (10 M, 1 mL) was added to a mixture of 2 (0.30 g) and dithiothreitol (0.77 g) in 10 mL of DMSO under nitrogen atmosphere, and the reaction mixture was stirred at room temperature for 3 min. 1-Propanol (30 mL) was added, and the reaction mixture was stored at 4 °C for 1 hr. The white precipitate obtained by centrifugation (1,500 x g, 10 min) was resuspended in 1-propanol (30 mL), and the suspension was subjected to centrifugation (1,500 x g, 10 min) to remove the supernatant. This process was repeated twice. The resulting precipitate was dried in vacuo to obtain a white powder of purified trisodium selenophosphate (190 mg, y. 78%). The chemical purity of the product was confirmed by 31P NMR, 77Se NMR, and ESI-MS analyses. 31P NMR (243 MHz, Tris-HCl buffer, pH 7.2) δ 23.5 (s, relative intensity was 0.92), δ 23.5 (d, relative intensity was 0.08, J31P-77Se = 538 Hz). 77Se NMR (114 MHz, Tris-HCl buffer, pH 7.2) δ −177.2 (d, J77Se-31P = 538 Hz). HRMS (ESI) calcd. for H4O3PSe+ [M+H]+: 162.9058, found: 162.9062.
LC-ICP-MS/MS analysis
An Agilent 8800 ICP-MS/MS (Agilent Technologies, Hachioji, Tokyo, Japan) was used. The operating conditions are summarized in Table 1 . The ICP-MS/MS was coupled to an HPLC system as the detector for the simultaneous speciation of Se and P. The HPLC system consisted of an on-line degasser, an HPLC pump (Prominence, Shimadzu, Kyoto, Japan), a Rheodyne six-port injector with a 20 µL sample loop, and a column. A multi-mode gel filtration column, Shodex Asahipak GS-320HQ (7.5 i.d. x 300 mm, with a guard column, 7.5 i.d. x 75 mm, Showa Denko, Tokyo, Japan), was used. The column was injected with a 20-µL aliquot of sample and then eluted with 50 mmol/L ammonium acetate, pH 6.5, at the flow rate of 0.6 mL/min. The eluate was introduced directly into the nebulizer of the ICP-MS/MS, and Se and P distributions were monitored at m/z 94 and 47 under the O2 mass shift mode as SeO+ and PO+, respectively.
| Plasma setting | |
|---|---|
| RF power (W) | 1,550 |
| Nebulizer type | MicroMist |
| Nebulizer gas flow (L min-1) | 0.90 |
| Make-up gas flow (L min-1) | 0.25 |
| Plasma gas flow (L min-1) | 14.0 |
| Reaction/Collision cell | |
| O2 gas flow (mL min-1) | 0.3 |
| Data acquisition | |
| m/z monitored | 94 shifted from 78 for Se as 78Se16O+
47 shifted from 31 for P as 31P16O+ |
Synthesis and identification of selenophosphate
Chemically synthesized selenophosphate was obtained on the basis of the synthetic sequence shown in Scheme 1. Selenophosphate precursor 2 protected by trimethylsilyl groups was generated by the direct oxidation of 1 using elemental Se, and selenophosphate was obtained as a trisodium salt by the hydrolysis of 2 under basic condition. Each product was appropriately identified by NMR and ESI-MS measurements, as described in Materials and methods.

Synthesis of trisodium selenophosphate.
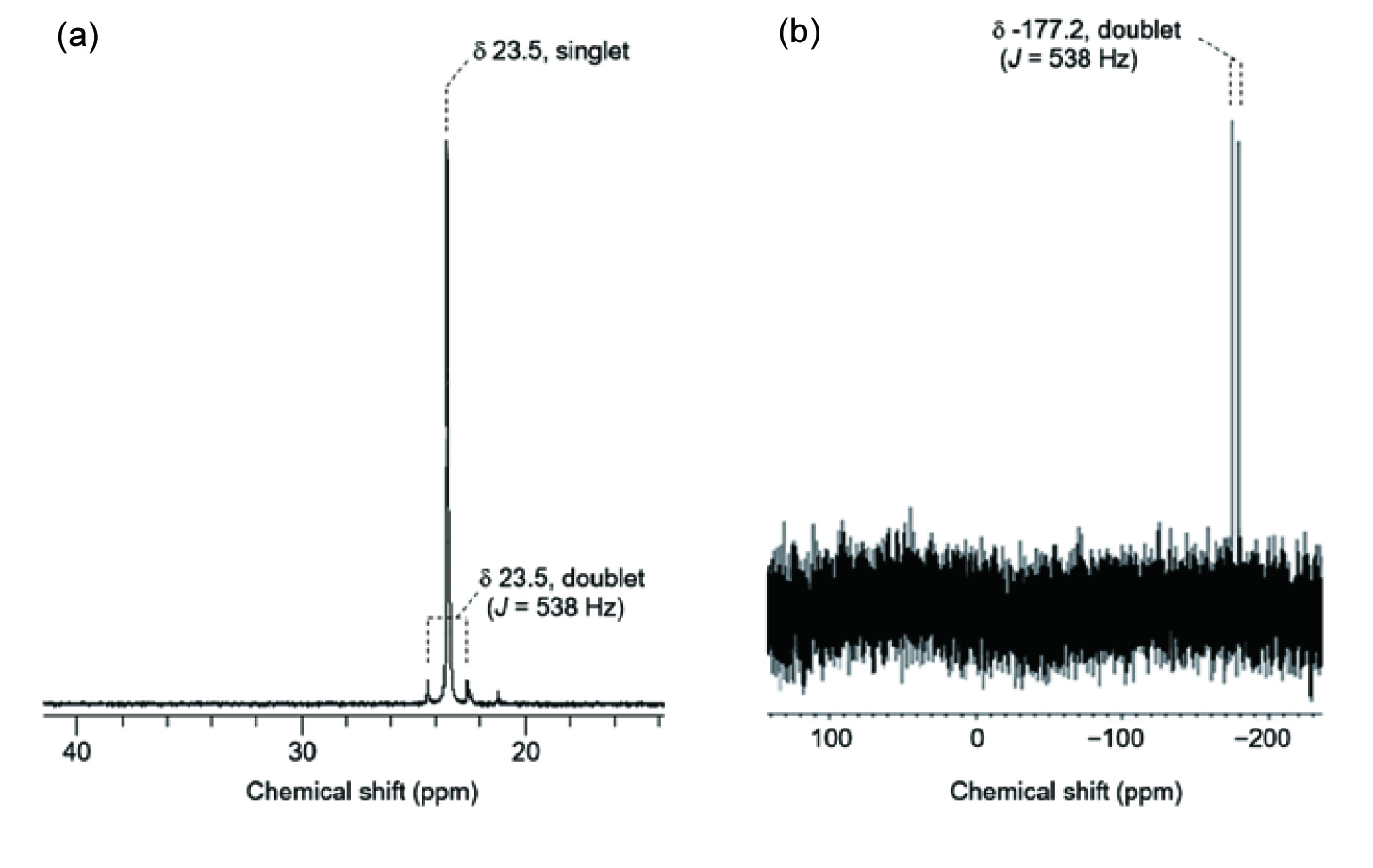
NMR spectra of selenophosphate in Tris-HCl buffer (100 mM, pH 7.2). (a) 31P NMR spectrum of purified trisodium selenophosphate (3 mM). (b) 77Se NMR spectrum of purified trisodium selenophosphate (200 mM).
The 31P and 77Se NMR spectra of chemically synthesized selenophosphate in Tris-HCl buffer are shown in Figure 1 . The chemical shift of 31P in this compound was consistent with that of the selD product previously reported by Stadtman’s group [19]. The 31P spectrum in Figure 1(a) contains a singlet with a relative intensity of 0.92, and a doublet with a relative intensity of 0.08 derived from the coupling with 77Se nuclei. This doublet demonstrated the presence of 77Se adjacent to the 31P nuclei, and the relative intensity of this signal was consistent with the natural abundance of 77Se (7.6%). The 77Se spectrum in Figure 1(b) shows a doublet derived from the coupling with 31P nuclei. Because the coupling constants of these doublets matched (J31P-77Se = 538 Hz), the existence of a Se=P double bond was confirmed.
Speciation of chemically synthesized selenophosphate
Chemically synthesized selenophosphate was subjected to LC-ICP-MS/MS analysis. Se and P in the chemically synthesized selenophosphate were simultaneously eluted on the GS-320HQ column at the retention time of 14.5 min, and no other Se and P peaks were detected ( Figure 2 ). We have reported the retention times of many low molecular weight selenium metabolites of microorganisms, plants, and animals under the same elution conditions. However, none of the retention times of the hitherto reported selenometabolites matched the unique retention time of Se and P. Based on the peak heights, the Se/P molar ratio at the retention time of 14.5 min was 0.91 ± 0.05. Phosphate was eluted at the retention time of 14.7 min under the same conditions as those for selenophosphate (data not shown), indicating that the compound composed of Se and P at the molar ratio of approximately 1 had a similar molecular structure to phosphate according to the chromatographic behavior. Hence, we conclude that highly purified selenophosphate was successfully synthesized on the basis of LC-ICP-MS/MS data. As red elemental Se appeared in the solution of the chemically synthesized selenophosphate during 48-hr preservation, we evaluated the stability of the selenophosphate under ambient condition.
Under ambient condition for 48 hr, the peak height of selenophosphate at the retention time of 14.5 min was markedly decreased to 2.6% of the original height ( Figure 3 ). In addition to the decrease in the peak height of selenophosphate, the peak at the retention time of 16.2 min was increased in the 48-hr selenophosphate solution. Selenate and selenite authentic standards were detected at the retention times of 14.0 and 16.2 min, respectively. The oxidation numbers of Se in selenophosphate, elemental Se, and selenite are -II, 0, and +IV, respectively. Hence, selenophosphate was decomposed, namely, oxidized to form elemental Se and selenite under ambient condition. These results indicated that selenophosphate was susceptible to oxidation under ambient condition, and this susceptibility seemed to contribute to its being a selenium donor in the de novo synthesis of selenocysteine.
In conclusion, chemically synthesized selenophosphate was subjected to detailed structural analyses by 31P and 77Se NMR and LC-ICP-MS/MS measurements in comparison with the reported data of the enzymatic product, and as a result, the structure of the SelD product was confirmed.

Elution profiles of Se and P in the solution of chemically synthesized selenophosphate. A 20-µL aliquot of the solution was injected into a GS-320HQ column, and the eluate was monitored for Se and P by ICP-MS/MS at m/z 94 and 47 as 78Se16O+ and 31P16O+, respectively.

Elution profiles of Se in the solution of chemically synthesized selenophosphate and inorganic Se authentic standards. A 20-µL aliquot of the solution of selenate (a), selenite (b), chemically synthesized selenophosphate immediately after dissolution (c), and chemically synthesized selenophosphate 48 hr after dissolution (d), each at the concentration of 1,000 ng/mL was injected into a GS-320HQ column, and the eluate was monitored for Se by ICP-MS/MS at m/z 94 as 78Se16O+.
This study was supported by grants from JSPS KAKENHI (Grant Numbers 19H01081, 19H05772, and 21H04920).
The authors declare no conflict of interest associated with this manuscript.