2022 年 2 巻 2 号 p. rev-1-reg-11
2022 年 2 巻 2 号 p. rev-1-reg-11
Iron is an essential element for all living organisms. The ability of plants to absorb inorganic iron from soil is important not only for plants but also for mammals, which ultimately rely on plants as their nutrient source. In contrast to most plant species, Poaceae plants, including rice, have developed a distinct chelation strategy to efficiently acquire insoluble soil iron using iron-chelating substances such as mugineic acid (MAs), called phytosiderophores. Genes involved in the biosynthesis and transport of MAs and their resulting iron(III)-MA complexes across membranes have been identified. On the other hand, an efficient short-step synthesis of the substrates MA and 2′-deoxymugineic acid (DMA) has enabled a sufficient supply of these compounds. Furthermore, owing to the chemical synthesis of proline-2′-deoxymugineic acid (PDMA), a cost-effective analog of DMA, the effectiveness of phytosiderophores in promoting rice growth in alkaline soil has been demonstrated at an experimental field scale. Nicotianamine (NA), an MAs precursor essential for metal translocation within plant tissues, was recently shown to be absorbed as an iron(II) complex in the mouse small intestine by an amino acid transporter. The discovery of the biological role of NA in iron absorption by the small intestine not only highlights the biological significance of NA across the plant and animal kingdoms but also opens new possibilities for biofortification approaches. Here, we discuss the recent findings in MA research in terms of plant growth, application in agriculture, and the emerging nutraceutical value of NA in iron absorption in mammals.
Dcytb; Duodenal cytochrome B, DMA; 2′-deoxymugineic acid, DMT1; divalent metal transporter1, HEPH; hephaestin, HvYS1; Hordeum vulgare yellow stripe1, IRT1; iron-regulated transporter1, FPN1; ferroportin, FRO; ferric-chelate reductase, MA(s); mugineic acids, NA; nicotianamine, PAT1; proton-conjugated amino acid transporter1, PDMA; proline-2′-deoxymucinic acid, YS1/YSL; yellow stripe1/yellow stripe1-like, ZmYS1; Zea mays yellow stripe1
Iron is an essential mineral for redox reactions, electron transfer reactions, and enzymatic activity in living systems [1]. Considering the food chain, living organisms ultimately obtain the nutrient iron from plants [2, 3]. Therefore, the ability of plants to absorb iron through their roots is of great importance to the entire living world. The proportion of iron present in the Earth’s crust is approximately 5%, making it the fourth most abundant element after oxygen, silicon, and aluminum [4]; however, it is usually present in an oxidized, trivalent, water-insoluble form, which is not available for plants. Calculations show that for every unit increase in soil pH, the solubility of iron decreases by 1/1000 [5]. Therefore, approximately 30% of the worldʼs neutral to alkaline soils are iron-deficient for plants, making them unsuitable for farming [6]. Plants have developed two major strategies for efficient iron absorption: reduction and chelation (Figure 1) [7]. Plants generally reduce iron(III) (ferric iron) to iron(II) (ferrous iron) by the enzyme ferric-chelate reductase (FRO) [8] and taking up iron(II) through the iron-regulated transporter1 (IRT1) present in the root epidermis [9]. By contrast, Poaceae plants synthesize and secrete phytosiderophores such as mugineic acids (MAs) through TOM1 transporter [10] in response to iron-deficiency [7]. The secreted MAs form water-soluble complexes with iron(III), which are then absorbed through the yellow stripe1/yellow stripe1-like (YS1/YSL) transmembrane transporter [11].
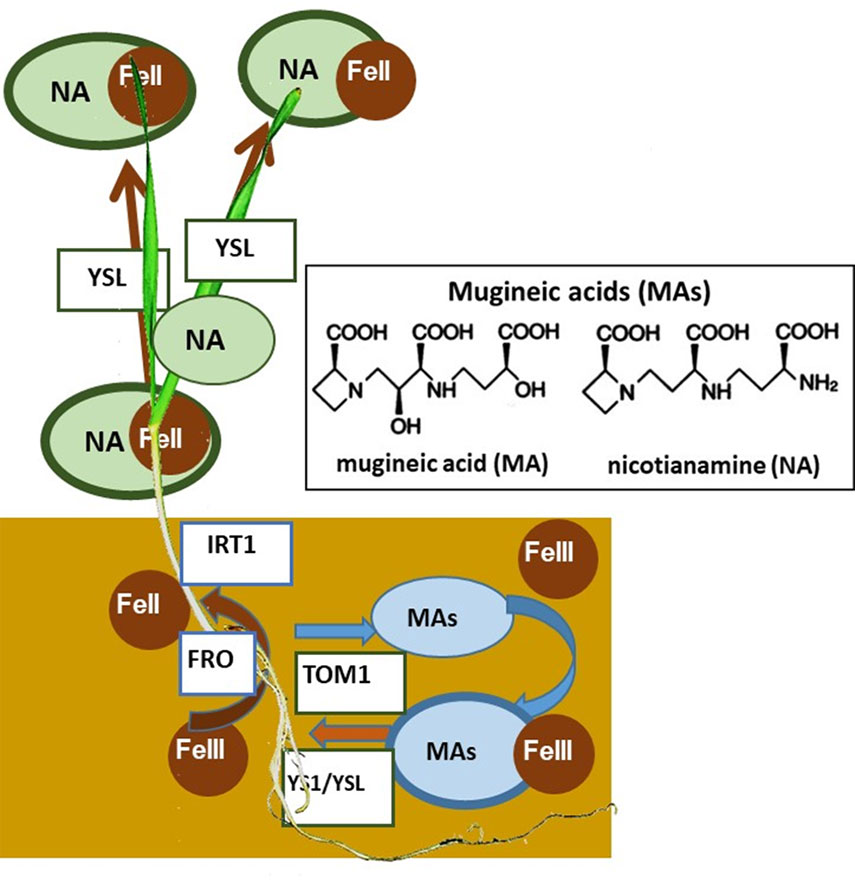
Plants other than those belonging to Gramineae reduce Fe(III) to Fe(II) by reductase ferric-chelate reductase (FRO) and absorb iron through the Fe(II) transporter iron-regulated transporter1 (IRT1). On the other hand, gramineous plants secrete mugineic acids (MAs) into the soil via the TOM1 transporter, and iron intake occurs through the MAs-Fe(III) complex, mediated by the specific transporter yellow stripe 1/yellow stripe1-like (YS1/YSL). Nicotianamine (NA), present in all plants, plays a role in iron transport by forming the NA-Fe(II) complex.
The structure of MA was first determined in 1978 by the Japanese group [12-14]. Takagi found that barley roots secreted iron-solubilizing substance from the roots in response to iron-deficiency [12]. Subsequently, Takemoto, Nomoto, and Takagi determined the structures of the substances and gave the name mugineic acids, which mean a kind of amino acid secreted from barley roots in Japanese [12, 13]. The biosynthetic pathway of MAs has been elucidated, in which L-methionine (Met) serves as a precursor for all known MAs [14-16], while Met is produced through the methionine cycle [15, 17]. All MAs share the same pathway from L-Met to 2′-deoxymugineic acid (DMA) via nicotianamine (NA), but the subsequent steps differ among plant species and cultivars. Most genes involved in phytosiderophore synthesis have been cloned, including those encoding adenine phosphoribosyl transferase (APRT), S-adenosylmethionine synthetase (SAMS), NA synthase (NAS), NA aminotransferase (NAAT), iron-deficiency-specific clones IDS2 and IDS3, and dioxygenases that hydroxylate the C-3 and C-2’ positions of MA [4,17-22]. All these genes were specifically induced in plant roots under iron deficiency conditions. Two cis-acting elements of the barley iron-deficiency-specific clone 2 (IDS2) promoter, the iron-deficiency-responsive elements 1 and 2 (IDE1 and IDE2), were involved in promoting iron-deficiency inducibility [23]. The secretion of phytosiderophores exhibits a distinct circadian rhythm [14]. The secretion time is likely controlled by the temperature around the roots [24]. The discovery of MAs by Japanese researchers has recently led to the elucidation of the mechanisms underlying iron absorption and iron transport in plants and mammals [25].
These studies establish that MAs are biologically important for grass plants and are biosynthesized at the expense of methionine through unique enzymatic reactions. However, since acquiring a large amount of naturally occurring MAs has been technically challenging, not much has been elucidated until recently as to whether the application of MAs to plants is sufficient for complementing the inhibited plant growth under iron-deficient conditions at the field experiment scale. Moreover, whether the phytosiderophores are utilized outside the plant kingdom has not been well understood. This review provides a brief overview of the effects of MAs on plant growth, possible agricultural impacts of the practical use of chemically synthesized MAs as ‘natural’ fertilizers, and the emerging nutraceutical value of NA in iron absorption in mammals.
Zea mays yellow stripe1 (ZmYS1) is a transporter of DMA-iron complexes that was first identified in 2001 using maize (Zea mays) mutant with yellow-white leaves (chlorosis) due to iron deficiency [26] ZmYS1 was reported to transport not only iron but also various metals complexed with DMA and the divalent NA-iron complex [27]. On the other hand, the absorption of metal complexed with MA in barley (Hordeum vulgare) was studied using various radio isotope-labeled metals. It was found that iron complexes were specifically absorbed [28]. Therefore, barley seemed to have a transporter with a substrate selectivity different from that of maize. We isolated and identified a 2430 bp (678 amino acid) long cDNA of the barley transporter Hordeum vulgare yellow stripe1 (HvYS1), which shares 72.7% similarity with ZmYS1 [29]. Focusing on the sixth extramembrane loop with low homology of the amino acid sequences of HvYS1 and ZmYS1, about 40 amino acid residues in this region were analyzed by AGADIR software and CD measurements. The results showed that HvYS1 has an α-helical structure, whereas ZmYS1 has a random structure. HvYS1 and ZmYS1 chimeras containing these 40 amino acid residues were expressed in Xenopus oocytes and their transport activity was measured. These studies indicated that the sixth extramembrane loop was responsible for the substrate specificity of HvYS1 [30].
In addition to HvYS1 and ZmYS1, further studies have identified 18 and 5 YSL genes in maize and barley, respectively. Moreover, YSL family genes with amino acid sequence homology of approximately 60% have been identified in various plant species, including 18 from rice (Oryza sativa), eight from Arabidopsis thaliana, six from grape (Vitis vinifera), three from the metal-accumulating plant Thlaspi caerulescens, two from Physcomitrella patens, and 18 from Brachypodium distachyon [11,31,32]. The substrates transported by these YSLs have been reported and are summarized in Table 1 [26, 27, 29, 33-43]. The sequence similarity between these YSL transporters and HvYS1 within the 40 amino acid residues in the sixth extramembrane loop is relatively low. OsYSL15 [36, 37] and OsYSL18 [38], which transport the iron-MA complex in rice, as well as HvYS1, had the highest α-helix percentage of 17.1%. However, recent studies have demonstrated that all functionally characterized YSL transporters in A. thaliana, which do not biosynthesize MAs, exclusively transport NA-metal complexes instead of MA-metal complexes [39-41, 43]. In fact, this is characteristic of YSL transporters that transport NA-metal complexes in the above-ground parts of plants. One example is the OsYSL2, which is expressed in the above-ground part of rice and transports Fe(II) and Mn(II) complexed with NA but not with MA [35]. Elucidating the factors that determine the metal complex transport selectivity of this YSL transporter is expected to lead to the prediction and modification of substrate selectivity in the future.
The genes participating in the biosynthesis of MAs in rice have been identified [19, 21, 44], thus raising the possibility of engineering rice plants that can acquire iron more efficiently. Rice has a lower resistance to iron-deficiency stress because it secretes a small amount of MAs than barley. On the other hand, when rice is grown under flooded conditions, iron-deficiency may not be a problem due to soil reduction. Furthermore, under flooded conditions, secretion of MA is not an efficient way to acquire iron due to diffusion of MA in soil solution. In rice plants that are easily transformed, resistance to salty alkaline soil was acquired when MA synthesis was enhanced or a proof of concept experiment was conducted. We are interested in whether it is more effective in the state of upland rice. The amount of MA secretion follows the order: barley > wheat, rye ≫ corn ≫ sorghum ≫ rice [45]. Transgenic rice carrying the barley genes involved in MAs synthase, secretion, and transport has been reported to be tolerant of iron deficiency in alkaline soil [25, 46-48]. Hence, if MA can be easily synthesized, it is expected to be a useful fertilizer. MAs are amino acid derivatives consisting of three reductively coupled units of azetidine carboxylic acid, aspartic acid, and malic acid. The efficient coupling of these units is the key to their synthesis. Although many MA and DMA synthesis methods were reported [49-58], multiple steps were required to desorb the protecting group of each amino acid unit in all these methods. Therefore, we improved the synthetic route based on the new concept of minimizing the use of amino acid protecting groups and minimizing the isolation and purification of each synthetic intermediate, establishing a continuous one-pot coupling method in which unprotected amino acids and aldehyde equivalents are added one after another [59]. This simplified synthetic method afforded DMA in yields up to 55% from N-Boc-L-allylglycine and enabled many synthetic MAs for mechanistic studies of phytosiderophores. So far, this method has been further improved for supply of low cost analog of DMA, PDMA, on a large scale (vide infra) [60]. In addition, a unified approach to natural phydosiderophores was recently reported by Stanetty et al [61].
| plant | YS1/YSL transporter |
substrate complex | No or weak substrate activity | method | Reference No. |
|
|---|---|---|---|---|---|---|
| PS or NA | metal | metal | ||||
| Zea mays (corn) |
ZmYS1 | DMA(PS) | Fe(III)- | yeast | 26 | |
| ZmYS1 | DMA(PS) | Fe(III)-, Cu(II)- | Zn(II)- | yeast | 33 | |
| NA | Fe(III)- | Fe(III)-, Zn(II)-, Cu(II)- | ||||
| ZmYS1 | DMA (PS) | Fe(III)-, Fe(II)-, Zn(II)-, Cu(II)-, Ni(II)-, Mn(II)-, Cd(II)- | oocytes, yeast | 27 | ||
| NA | Fe(III)-, Fe(II)-, Ni(II)- | |||||
| ZmYS1 | DMA (PS) | Fe(III)-, Zn(II)-, Cu(II)-, Ni(II)-, Mn(II)-, Co(II)- | oocytes, yeast | 29 | ||
| NA | Fe(II)- | |||||
| Hordeum vulgare (barley) |
HvYS1 | MA(PS) | Fe(III)- | Fe(II)-, Zn(II)-, Cu(II)-, Ni(II)-, Mn(II)-, Co(II)- | oocytes, yeast | 29 |
| DMA(PS) | Fe(III)- | |||||
| NA | Fe(II)- | |||||
| HvYSL2 | DMA (PS) | Fe(III)-, Zn(II)-, Cu(II)-, Ni(II)-, Mn(II)-, Co(II)- | oocytes | 34 | ||
| NA | Fe(II)- | |||||
| Oryza sativa (rice) |
OsYSL2 | DMA (PS) | Fe(III)-, Mn(II)- | oocytes | 35 | |
| NA | Fe(III)-, Mn(II)- | Fe(III)-, Zn(II)-, Cu(II)- | ||||
| OsYSL15 | DMA (PS) | Fe(III)- | oocytes, yeast | 36 | ||
| NA | Fe(III)-, Fe(II)-, Mn(II)- | |||||
| OsYSL15 | DNA (PS) | Fe(III)- | yeast | 37 | ||
| NA | Fe(II)- | |||||
| OsYSL18 | DMA (PS) | Fe(III)- | Zn(II)- | oocytes | 38 | |
| NA | Fe(II)-, Zn(II)- | |||||
| Arabidopsis Thaliana |
AtYSL1 | NA | Fe(II)- | mutants in Arabi. | 39 | |
| AtYSL2 | MA(PS) | Fe(II)-, Fe(III)-, Cu(II) | yeast | 40 | ||
| NA | Fe(II)-, Cu(II)- | Fe(III)-, | ||||
| AtYSL2 | NA | Fe(II)-, Fe(III)-, Ni(II) | yeast | 41 | ||
| AtYSL3 | NA | Fe(II)- | mutants in Arabi. | 42 | ||
| Thlaspi caerulescens (metal accumulatrion plant) |
TcYSL3 | NA | Fe(II)-, Ni(II)- | yeast | 43 | |
PS; phytosiderophore
DMA; deoxymugineic acid
MA; mugineic acid
NA; nicotianamine
Synthetic MAs seem to have a beneficial effect in approving iron-deficiency stress in rice [62, 63]. The hydroponic cultures of rice (Oryza sativa) seedlings show almost complete restoration in shoot height and soil-plant analysis development (SPAD) values after treatment with 3–30 µM DMA at high pH (pH 8.0), compared with untreated control seedlings at normal pH (pH 5.8). Surprisingly, DMA application also increased nitrate reductase activity and the expression of genes encoding high-affinity nitrate transporters and nitrate reductases, all of which were otherwise considerably lower under high pH conditions. These data suggest that exogenous DMA not only plays a vital role in facilitating the uptake of environmental iron but also orchestrates iron and nitrate assimilation for optimal growth under high pH conditions. Since MAs showed better growth improvement than other synthetic iron chelators, such as EDTA, MAs may have potential for field application in alkaline soil [62, 63].
The efficient synthesis of DMA made it possible to conduct soil experiments with the addition of DMA. However, the instability of the four-membered ring of DMA and the extremely high cost of L-azetidine-2-carboxylic acid, the raw material for this four-membered ring, become a major limiting factor for the practical use of DMA as a fertilizer. Therefore, a recent study synthesized various analogs of L-azetidine-2-carboxylic acid substituted with stable and inexpensive amino acids and evaluated the intracellular transport activity of their trivalent iron chelates using insect cells expressing HvYS1. Results showed that PDMA, i.e., DMA modified with L-proline had an iron complex transport activity similar to DMA [60]. Because L-proline is a natural amino acid available at a low cost, the synthesis cost of PDMA was reduced to 1/1000–1/10000 in comparison to DMA, thus solving the raw material cost problem. Furthermore, while soil microorganisms degrade natural MAs in 1 day, PDMA takes approximately one month to be degraded, thus maintaining its effectiveness over a long time. Because PDMA is degradable, it can be used as an environmentally friendly fertilizer. Therefore, an improved synthetic method suitable for the quantitative synthesis of PDMA was developed based on the above synthesis of DMA [60], and a trial was conducted. The results showed that PDMA was approximately ten times more effective than the existing iron chelators in restoring iron deficiency in alkaline soil (Figure 2) [60].

(A) Structure of synthetic DMA analog PDMA. (B) Four-week-old rice plants were treated with 30 μM PDMA (left) or without PDMA (right). (Provided by Dr. Motofumi Suzuki, Aichi Steel Corporation)
Iron deficiency, including severe anemia, occurs worldwide and therefore effective remedies are desired. People in Southeast Asia, including the Japanese, consume more than 85% of the iron in the form of non-heme iron from plant foods [64, 65]. Humans ultimately depend on crops for iron supply; therefore, iron availability to crops is essential [66]. Iron forms a complex with NA that transports it mainly to plant stems, leaves, flowers, seeds and fruits. Activation of OsNAS, which encodes rice NA synthase [22], leads to an increase in iron concentration in the leaves and seeds of rice [67-69]. Recently, it was reported that hemoglobin levels increased in mice fed with transgenic rice overexpressing NA synthase [70]. The authors concluded that increasing the NA amount in transgenic rice also increased iron content, resulting in a higher iron in mice than in those fed with wild-type rice. On the other hand, soybean (Glycine max) grains and soy sauce contain a large amount of NA, which is an inhibitor of angiotensin-converting enzymes and then decreases blood pressure in hypertensive mice [71-73]. A recent study with quantitative analysis of NA in plasma showed that it was not decomposed after digestion but carried into the blood [72, 73]. Therefore, it seems that iron-chelating compounds produced by soybean, such as NA, are involved in iron absorption and transport in humans.
Iron absorption in humans mainly occurs in the duodenum; iron is absorbed as heme iron from animal foods and as inorganic iron from plant foods [74]. Since inorganic iron exists in a trivalent state, it is reduced to divalent iron by the reductase duodenal cytochrome B (Dcytb) [75] present in the small intestinal lumen. It is transported into the cell via the divalent metal transporter1 (DMT1) [76, 77]. The incorporated iron is stored as ferritin or transported via ferroportin1 (FPN1) on the basolateral cell membrane [78-80]. It is then oxidized by hephaestin (HEPH) to form iron(III), which binds to transferrin [81]. The mammalian absorption mechanism of inorganic iron or nonheme iron, which is abundant in plant foods uses the Dctyb/DMT1 pathway (reducion to divalent iron by Dcytb and transport into the cell via DMT1) in the duodenum [74] (Figure 3).
It has been reported that Dcytb is not essential in mice because knocking out this gene from small intestinal epithelial cells did not lead to iron deficiency [82]. Therefore, it is suggested that there is an iron absorption mechanism that is not explained yet and that NA, which is essential for iron transport in plants, is also involved in iron transport between cells in mammals. Since the NA-Fe(II) complex transporters YS1/YSL of plants belong to a family of oligopeptide transporters [83, 84], they possess trans oligopeptides and amino acids from the solute carrier (SLC) family involved in absorption in the small intestine [85, 86]. Through screening of transporters, a proton-conjugated amino acid transporter1 (PAT1; SLC36A1) was found in the small intestinal epithelial cells [87] and showed a transport activity similar to that of the NA-iron(II) complex (Figure 3) [88]. PAT1 was expressed in Xenopus oocytes for its electrophysiological activity measurement, revealing that NA-Fe(II) is transported by PAT1. In addition, NA-59Fe(II) oral administration in mice showed a high iron intake 30 min after administration in the proximal jejunum, where PAT1 expression was also observed. In contrast, when free 59Fe(II) was administered, iron absorption and DMT1 expression were observed in the duodenum, indicating that free iron has an absorption site different from that of the NA-iron(II) complex (Figure 3) [88, 89]. Furthermore, when comparing NA-59Fe(II) and 59Fe(II) 5 h after administration to mice, NA-59Fe(II) complex administration resulted in a higher absorption rate of 59Fe in the spleen and kidney. The ferrous iron was given with a high dose of ascorbic acid; absorbed ferrous iron without NA can cause intravascular hemolysis and/or hemorrhagic gastric ulcer via Fenton reaction. Subsequently, hemoglobin level was decreased in mice given ferrous iron without NA. On the other hand, NA may have prevented the hemolysis, and the hemoglobin level was not changed or a little increased from the baseline. These results probably demonstrate the effect of NA on iron absorption in mice [88]. Further comparison of long-term administration experiments in mice of iron or NA-iron complex is required.

In mammals, iron ingested from plant food is reduced to ferrous iron in the duodenum by duodenal cytochrome B (Dcytb) and is absorbed by the divalent metal transporter1 (DMT1). (A) The incorporated iron is transported via ferroportin (FPN1) and oxidized by hephaestin (HEPH). (B) It has been revealed that the nicotianamine (NA)-Fe(II) complex is absorbed by the amino acid transporter proton-conjugated amino acid transporter 1 (PAT1) in the proximal jejunum. It is undecided whether the incorporated NA-Fe(II) complex is transported as it is or as free iron by FPN1.
Here, we explained the absorption mechanism of mugineic acid in plants and its application, and the iron absorber in the small intestine by the precursor nicotianamine of mugineic acid in plant foods. Research on MAs and their analogs will contribute to solving the problem of food shortages caused by population growth and to the greening of poor soil on a global level. On one hand, MAs could be used to develop environmentally friendly fertilizers of iron. On the other hand, NA is useful for iron uptake and the efflux of excess iron in mammals. Free iron elicits a Fenton reaction, which sometimes causes ferroptosis [90] implicated in pathological cell death associated with degenerative diseases (e.g., Alzheimerʼs, Huntingtonʼs, and Parkinsonʼs diseases), carcinogenesis, stroke, intracerebral hemorrhage, traumatic brain injury, ischemia-reperfusion injury, and kidney degeneration [91]. Therefore, NA may be preferable for use in chelation therapy. The molecular mechanism that regulates iron absorption by nicotianamine remains unclear, and the regulatory mechanism of iron complex transporters in the small intestine needs to be elucidated. Further research revealing these points provides new insights for improving iron nutrition and contributes to human health. Generally, there are two main strategies for iron acquisition in biological organisms: reduction and chelation. Bacteria are known to produce siderophores that form complexes with Fe(III). Recent studies have identified as mammalian siderophore a low-molecular-weight 2,5-dihydroxy benzoic acid with similarities to the 2,3-dihydroxy benzoic acid found in bacterial siderophores [92]. Staphylococcus aureus has been shown to biosynthesize staphyropine, which is an NA-like metallophore that forms a complex with Fe(II) [93, 94] (Figure 4). The structure of bacterial siderophores and respective transporters are versatile in general. However, the discovery of NA-like staphyropine from bacteria and the putative function of NA in the small intestine suggests the functional and structural convergence of NA as an iron chelator across three kingdoms.

There are two main strategies for iron acquisition in biological organisms: reduction and chelation. Plants have FRO/IRT1 and phytosiderophores systems. Bacteria are known to produce siderophores that form complexes with Fe(III) for example enterobactin. Staphylococcus aureus has been shown to biosynthesize staphyropine, which is an NA-like metallophore that forms a complex with Fe(II) recently. Mammals have iron uptake system in intestine; Deytb/DMT1 uptake Fe(III) and heme transport heme (Fe(II)) in duodenum and PAT1 transport NA-Fe(II) in the proximal jejunum. Recent studies have identified the mammalian siderophore as a low molecular weight 2,5-dihydroxybenzoic acid similar to the 2,3-dihydroxybenzoic acid found in the bacterial siderophore. Bacterial, plant and mammalian siderophores are becoming known to have similar iron absorption mechanisms.
We thank Dr. Hiroyuki Kimura and Dr. Hidekazu Kawashima from the Kyoto Pharmaceutical University for using mice and radioisotopes at the Radioisotope Research Center. This work was supported by JSPS (KAKENHI) Grant Numbers JP25350973 and JP16K01927.
The authors declare no conflict of interest associated with this manuscript.