2024 Volume 11 Pages 33-36
2024 Volume 11 Pages 33-36
Spinal cord stimulation (SCS) is widely performed to treat several types of intractable chronic pain. To maintain lasting SCS, epidural electrode leads must be replaced sometimes due to problems like lead breakage. However, in lead replacement, guiding the new lead to the original position may be difficult because granulation tissue sheath forms around the lead. We encountered a surgical case where we inserted new leads through tissue sheaths forming around the old leads from the epidural space to the thoracolumbar fascia; the lead was smoothly introduced to the original place. This procedure is simpler than previously reported techniques. Here, we report the detailed surgical procedure and review the relevant literature.
An early type of chronic spinal cord stimulation (SCS) for pain with epidural electrode leads has been used since the 1970s in Japan, and the current type of SCS for chronic intractable pain was approved for insurance coverage in 1992. The implantable pulse generator (IPG) and electrode lead have improved with technological advancements, but SCS therapy was not approved for magnetic resonance imaging (MRI) use until approximately a decade ago. The MRI-compatible SCS device was launched commercially in 2012,1) and the full-body MRI-compatible SCS system was introduced in Japan in 2014.
To maintain a lasting SCS, partial or complete system replacement is sometimes required because of device failure or MRI-related problems. Epidural electrode lead replacement is required when the lead breaks and patients request a change from a non-MRI-compatible system to an MRI-compatible one.
However, during lead replacement surgery, guiding the new lead to the original position may be difficult since a granulation tissue sheath has already formed around the lead.2,3) This may reduce its efficacy, leading to insufficient pain control. Nevertheless, there are few technical reports on lead replacement in SCS, but no established surgical methods exist.
We discussed a surgical case where we inserted a new lead through the tissue sheaths. The sheaths were tough and formed around the old leads from the epidural space to the thoracolumbar fascia; the lead was smoothly introduced to the original place. Here, we report the detailed surgical procedure and review the relevant literature.
Informed consent was obtained from all patients, and this retrospective study was approved by the Institutional Review Board of Kameda General Hospital (No. 23-005).
The patient was a 45-year-old woman who underwent foramen magnum decompression in her 20s for dysesthesia in the upper and lower extremities caused by Chiari malformation type 1 with syringomyelia. Nevertheless, severe neuropathic pain in the lower extremities persisted after the surgery, and SCS was introduced nine years ago, which has been effective. The stimulation system contained non-MRI-compatible devices, such as RestoreSensor, Octad leads, and extension leads (Medtronic, MN, USA).
The patient requested to exchange the old devices for a full-body MRI-compatible system when implantable pulse generator (IPG) exchange was required due to its nine-year battery life after SCS introduction.
Two leads were inserted from the L2/3 interlaminar to the epidural space, and the tips of the leads were placed at the T7 vertebral level (Fig. 1). SCS-induced paresthesia covered the painful areas in the lower extremities, and no pain was observed in the follow-up period.

In the preoperative spinal plain radiograph, the epidural electrode tip is located at the 7th thoracic vertebra (A, B).
We considered the lead location adequate, and guiding the new leads to the same location was optimal for total SCS system replacement.
Operative procedureSurgery was performed under general anesthesia, with the patient placed in the prone position. We reopened the previous skin incision in the lumbar region and traced the leads running subcutaneously to the thoracolumbar fascia penetrating point, confirming the tunnels of granulation tissue around the leads at the point: tissue sheaths (Fig. 2). The tissue sheaths formed tracts reaching the epidural space along the leads.
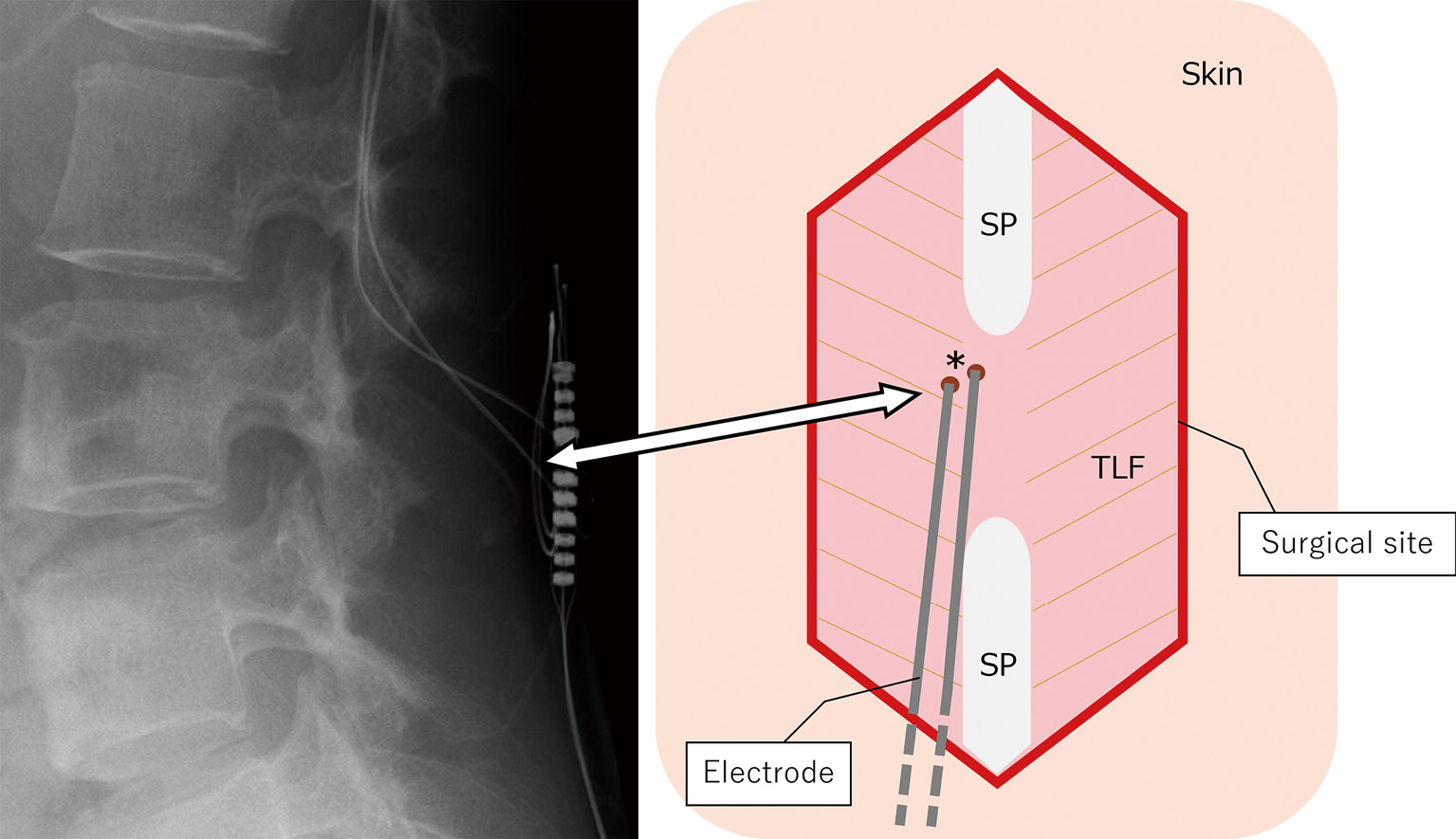
An X-ray image and a schema of the surgical site showing the entry points of tracts penetrating thoracolumbar fascia to epidural space (*). SP: spinous process, TLF: thoracolumbar fascia
We carefully removed the old lead smoothly and inserted a new one, Vectris SureScan MRI (Medtronic, MN, USA), with a vent stylet into a tract formed around the old lead. The new lead was smoothly guided to the original epidural position. Torque was not required for epidural lead guidance. The lead advancement route was confirmed using intraoperative fluoroscopy (Fig. 3). The lead was moved rostrally and caudally within the epidural space several times and guided to the same course every time. Another lead was replaced in a similar manner. Two leads were fixed, leading to the fascia and passing the proximal sides of the leads subcutaneously toward the right buttock. We removed the old IPG and implanted a new IPG, Intellis (Medtronic, MN, USA), subcutaneously into the right buttock after connecting the entire system. Finally, the electrical resistance of the new SCS system was confirmed favorably.

Intraoperative X-ray images showing the electrode, steering through the tissue sheath in the epidural space.
Postoperative radiographs showed that the leads were in the original place (Fig. 4). The patient received the same stimulation effect as before the replacement surgery, and the pain did not recur until the last follow-up checkup.

Preoperative (A) and postoperative (B) X-ray images showing the electrode tips at the same place. T 7: 7th thoracic vertebra
We replaced the SCS leads with new ones, inserting them through the tissue sheaths formed around the old ones. The new leads were smoothly guided to the same tracts and placed simply in the original position.
While epidural granulation surrounding a lead can hinder lead replacement through a new puncture,2,3) Logé et al.3) reported that fibrosis surrounding the electrode that formed after some weeks or months guided the reinsertion of a new SCS lead. Regarding epidural catheters for pain therapy, it has been reported that epidural fibrosis, considered a foreign body reaction, developed within 21 days and an average of 82.5 days.4) In this case, nine years have passed since SCS was introduced. Thus, this period was deemed sufficient for a tough tissue sheath to develop around the lead. Combined with previous reports3,4) and our experience, we speculate that if several months have passed since SCS introduction, lead replacement through a tissue sheath, as described above, is achievable.
Technical reports on reinsertion without a new puncture and guiding a new SCS lead unobstructed by epidural granulation are few. Matsumura et al.2) reported a case where the ligamentum flavum was opened, a Tuohy needle was inserted into the epidural space guided by old epidural electrodes, and new electrodes were placed in the previous position. There are two technical reports of epidural electrode replacement where an introducer sheath was inserted into the epidural space guided by the old epidural electrode.5,6) A method reported by Logé et al.3) was similar to ours; however, they inserted leads through tissue sheaths by opening the interspinous ligament. Compared to previous reports, our procedure is simpler because it can be completed by manipulating the layers superficial to the thoracolumbar fascia. Logé et al.3) reported that the success rate of the technique was high (11/14 patients), but no consideration was given to why the three procedures were impossible.
In this case, we exchanged two Octad leads with Vectris SureScan MRI leads (Medtronic, MN, USA). The diameters of both leads were 1.3 mm, which may have been an important element in the success of the procedure. However, if the diameter of the new lead had been larger than that of the old lead, the procedure may be impossible. The lower tortuosity of the lead tract may also be another element, and it is speculated to be difficult to insert and guide a lead into the winding tract of the epidural space. Moreover, we think that the Vectris SureScan MRI lead (Medtronic, MN, USA) is harder than other manufacturers' products, possibly contributing to the procedure's success. However, in either case, preparing a new puncture is recommended to perform this procedure. If the surgeon senses a resistance at the fingertips while inserting a lead into a tissue sheath, the procedure should be stopped, and a new puncture should be performed due to the risk of penetrating the dura mater with excessive force and damaging surrounding tissue.
In conclusion, we successfully replaced SCS leads with tissue sheaths formed around the old leads from the epidural space to the thoracolumbar fascia. This procedure is simpler than those described in previous reports. Since this is a case report, verifying whether the procedure can be repeated in the future procedures is necessary.
The authors have nothing to disclose.