2024 Volume 11 Pages 163-168
2024 Volume 11 Pages 163-168
Chronic encapsulated intracerebral hematoma is a rare type of intracerebral hemorrhage. Reportedly, it is associated with vascular malformations, including arteriovenous malformations, cavernous hemangiomas, microaneurysms, and venous malformations. Recently, an association between chronic encapsulated intracerebral hematoma and stereotactic radiosurgery for arteriovenous malformations has been reported. In general, as the hematoma enlarges, symptoms progress slowly. In this report, we present a case of a 50-year-old woman who had undergone clivus chordoma resection and carbon ion therapy for the clivus respectively 27 and 20 years before developing chronic encapsulated intracerebral hematoma with rapidly progressing disturbance of consciousness. She was referred to our hospital because of difficulty walking due to left hemiparesis. Head computed tomography and magnetic resonance imaging showed a cystic lesion in the right temporal lobe with perifocal edema. On the second day of hospitalization, the patient's consciousness worsened. We suspected a malignant glioma and performed an emergency craniotomy; however, the pathological diagnosis was chronic encapsulated intracerebral hematoma. After the rehabilitation therapy, the patient became ambulatory and was discharged. To the date of reporting, the patient remained recurrence-free. Chronic encapsulated intracerebral hematoma may be due to invasive craniotomy or carbon ion therapy. It usually progresses slowly; however, in some cases, such as this one, it may cause rapid deterioration of consciousness.
Chronic encapsulated intracerebral hematoma (CEIH) is a rare type of intracerebral hemorrhage that is reportedly associated with vascular malformations, including cerebral arteriovenous malformations (AVMs), cavernous hemangiomas, microaneurysms, and venous malformations.1-3) In recent years, an association of CEIH with stereotactic radiosurgery for AVMs has been reported.4,5) CEIH generally presents a gradual progression of symptoms as the hematoma enlarges over weeks.1,6) Carbon ion therapy is indicated for skull base tumors, such as chordomas and chondrosarcomas, in neurosurgery. It provides an excellent dose distribution and strong biological effects, thus exhibiting a strong cytocidal effect on X-ray-resistant tumors.7) Adverse events associated with carbon ion therapy include skin injury, mucosal injury, brain edema, optic nerve palsy, and brain necrosis.8) In rare cases, carbon ion therapy has caused cavernous hemangiomas and osteosarcomas,9) but no reports of CEIH caused by such therapy have been published. In this report, we present a case of a patient with CEIH who underwent clivus chordoma resection and carbon ion therapy respectively 27 and 20 years prior to experiencing rapidly progressing disturbance of consciousness after hospitalization.
A 50-year-old woman presented to our hospital with an impaired gait. She had undergone clivus chordoma resection and carbon ion therapy for recurrence respectively 27 and 20 years before developing chronic encapsulated intracerebral hematoma with rapidly progressing disturbance of consciousness. The carbon ion therapy was carried out at a dose of 57.6 Gr, delivered in 16 fractions, targeting the recurrent tumor extending from the middle cranial fossa (medial) to the clivus (Fig. 1). The patient had attended the hospital as an outpatient for 9 years following the therapy.

Pre-carbon ion therapy magnetic resonance images and the irradiation range of carbon ion therapy.
T1-weighted magnetic resonance imaging (MRI) reveals a low-intensity signal extending from the middle cranial fossa (medial) to the clivus (A, D), and the lesion is enhanced in T1-weighted enhanced MRI (B, E).
C and F show the planning of carbon ion therapy.
T1W1, T1-weighted image; GdT1W1, gadolinium-enhanced T1-weighted image
Upon admission, the patient's consciousness level was assessed using the Glasgow Coma Scale 14 (E4V4M6). Physical examination revealed right pupillary mydriasis (4 mm), loss of right contralateral light reflex, and left hemiparesis (manual muscle test score: 4). Computed tomography of the head revealed a cystic lesion in the right temporal lobe, with perifocal edema. T1-weighted magnetic resonance imaging (MRI) displayed a low-intensity signal and T2-weighted MRI exhibited a high-intensity signal within the cyst. The nodular lesion was heterogeneously enhanced with contrast medium inside the cyst. Fluid-attenuated inversion recovery MRI revealed markedly high intensity around the cyst, indicative of edema extending to the brainstem, and T2*-weighted MRI revealed a heterogeneous low-intensity signal within the nodular lesion (Fig. 2). These imaging findings led to the diagnosis of a brain tumor, possibly a malignant glioma.
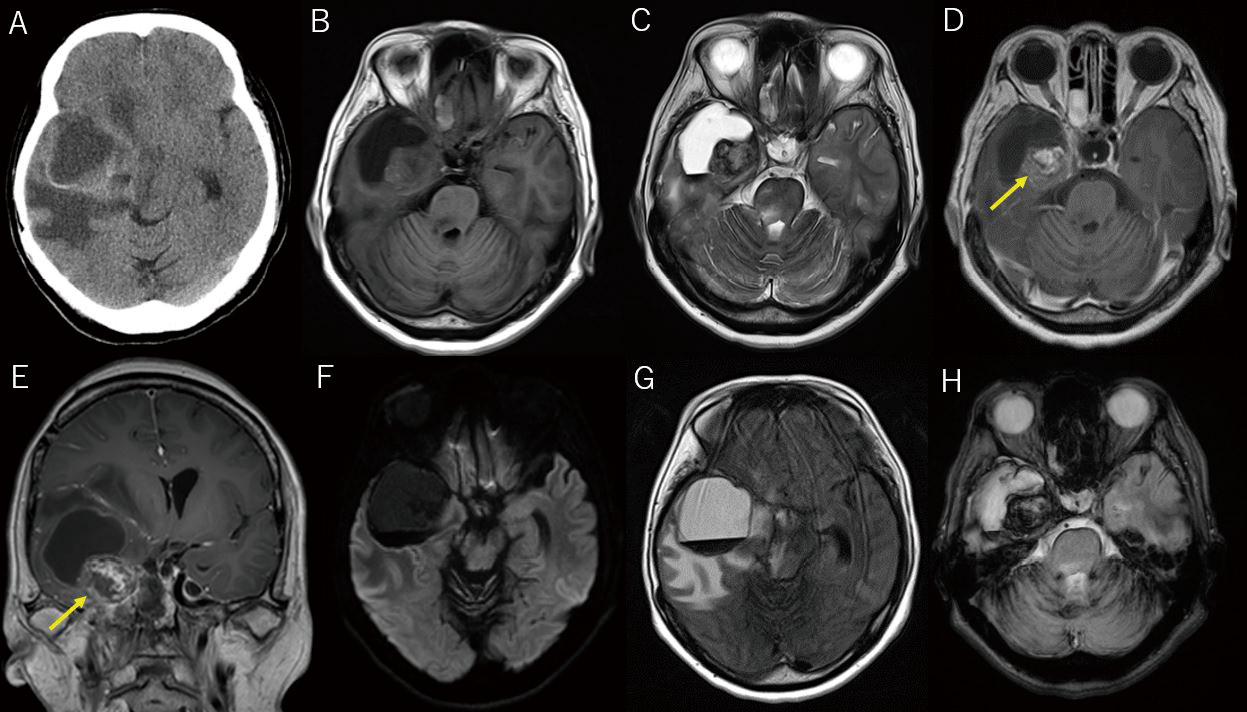
Radiographic images of the brain upon admission.
Computed tomography of the head reveals a cystic lesion in the right temporal lobe, with surrounding edema (A). T1-weighted magnetic resonance imaging (MRI) demonstrates a low-intensity signal (B), and T2-weighted MRI reveals a high-intensity signal in the cyst (C). T1-weighted enhanced MRI reveals a nodular lesion (arrow) inside the cyst with a heterogeneous contrast effect (D, E). Diffusion-weighted MRI does not show any high-intensity signal in the cyst (F). Fluid-attenuated inversion recovery MRI displays severe edema around the cyst, which extends to the brainstem (G). T2*-weighted MRI discloses a heterogeneous low-intensity signal within the nodular lesion (H).
On the second day of hospitalization, the patient's condition rapidly deteriorated; therefore, emergency surgery was conducted. To expose the right temporal lobe, we performed temporal craniotomy. Through the inferior temporal gyrus, we punctured the cyst and drained thin, brown, transparent fluid. Multiple small vessels were observed flowing into the nodular lesion from the surrounding brain tissue. Using a bipolar coagulation system, we were able to coagulate these vessels, achieving hemostasis without any difficulty. The nodular lesion was removed en bloc after carefully dissecting it from the normal brain tissue. Upon pathological examination, layered proliferation of hemosiderin-laden histiocytes was observed in the capsule, and hematoma formation with dilated small and large congregating vessels was observed in the nodular lesion, consistent with CEIH (Fig. 3). Hemangiomas were identified at the margins of the excised tissues. Additionally, clusters of foam cells reminiscent of radiation damage were observed around the hemangiomas (Fig. 3).

Pathological findings.
Pathological examination reveals an area (surrounded by a dotted line) of blood vessels of various sizes distended and filled with blood (A, B). Elastica-van Gieson staining reveals that the area is surrounded by elastic and collagenous fibers (arrows) (C, D), consistent with cavernous hemangioma. The area below it lacks elastic and collagen fibers and is believed to be a hemorrhage point (*) (C, D). Further down (*) (A, B), an area covered by stratified proliferation of hemosiderin-laden histiocytes (arrows) can be seen (A, B), and the interior contains a hematoma (arrows) and dilated small and large collecting vessels (arrowheads), with the appearance of an organizing hematoma (E). Within the cavernous hemangioma, an aggregation of foam cells is observed (arrows), suggestive of radiation injury (F).
The postoperative course was uneventful. The patient became ambulatory after the rehabilitation therapy and was then discharged. As of the reporting date, the patient remains recurrence-free (Fig. 4).

Postoperative magnetic resonance imaging.
Postoperative day 2 (A). One month after surgery (B). Four months after surgery (C).
Postoperative fluid-attenuated inversion recovery magnetic resonance imaging reveals that the edematous changes had decreased over time, with no evidence of cyst recurrence.
CEIH can occur after invasive surgery or carbon ion therapy. It was first reported by McKissock and Paterson in 195610) and described in detail by Hirsch et al. in 1981.11) To date, approximately 60 cases of CEIH have been reported,12) which accounts for 0.04% of all intracerebral hemorrhages. Its etiology includes vascular malformations,1-3) traumatic changes (including surgery),13) and radiotherapy for AVMs,4,5) although the cause often remains unknown.14) A systematic review revealed that in 48.3% of cases, the cause remains unidentified,12) suggesting underlying factors that are yet to be elucidated. The mechanism by which CEIH progresses may resemble that of a chronic subdural hematoma. During the initial hemorrhage of a chronic subdural hematoma, the cerebral tissue induces a fibroblast reaction, which forms an initial capsule membrane. Subsequently episodes of bleeding result in the production of granulation tissue, further stimulating fibroblastic reaction and the development of a fibrous capsule.12,13) Repeated bleeding occurs in the fibrous capsule because of a high level of angiogenesis and excessive activation of the fibrinolytic system. Subsequently, the hematoma expands as hematoma degradation products elevate the osmotic pressure within the hematoma, allowing cerebrospinal fluid to enter it.12,13) A possible mechanism for the development of CEIH after radiotherapy is that radiation may induce hypoxic stress on peri-brain tissues, triggering transcription of vascular endothelial growth factor (VEGF) and leading to abnormal angiogenesis and vascular leakage that enlarges the hematoma.4,5,15)
Carbon ion therapy is indicated for skull base tumors, such as chordomas and chondrosarcomas.16) Tumor control by carbon ion therapy offers advantages over conventional radiotherapy because a lower radiation dose is delivered to the healthy brain tissues surrounding the tumor. Charged particles deposit energy far more selectively than X-rays, allowing greater local control of the tumor and a lower probability of damage to healthy tissues in the treatment field (the Bragg peak).16) The reported complications include radiation necrosis, internal carotid artery occlusion, mucositis, dermatitis, and hearing loss.17) Although no reports of CEIH associated with carbon ion therapy have been published, given the mechanics of carbon ion therapy, the tumor may accumulate high energy doses, resulting in hypoxia in the irradiated area, inducing VEGF transcription and possibly causing CEIH. Although secondary cavernous hemangiomas and osteosarcomas have been reported in recent years,9) no reports of malignant gliomas due to carbon ion therapy exist. Additionally, due to the high selectivity of the carbon ion therapy irradiation range, carbon ion therapy may carry a lower risk of secondary cancers when compared with conventional radiation therapy.18) In the present case, these factors may help us suspect CEIH instead of a malignant glioma before surgery. Furthermore, because tissue resembling a cavernous hemangioma was observed at the margins of the organized hematoma, we suspected that the rupture of a cavernous hemangioma might have triggered the organized hematoma formation. Moreover, clusters of foam cells reminiscent of radiation damage were observed around the hemangioma, suggesting that the cavernous hemangioma might have been caused by carbon ion therapy. Compared with conventional radiotherapy, information on the long-term risk of complications due to carbon ion therapy is limited, and further case accumulation is desirable.
Imaging-based diagnosisPreoperatively, using imaging studies, distinguishing CEIH from malignant gliomas, metastatic tumors, and abscesses may be difficult.19) In cases of malignant gliomas, including glioblastomas, which are frequently encountered in daily practice, the cyst wall is frequently enhanced with contrast media, or the thickness of the cyst wall may be uneven. However, when CEIH exhibits a partially contrast-enhanced nodule, as in the present case, distinguishing it from malignant glioma remains challenging.
TreatmentTo treat CEIH, the capsule must be removed; this is because the capsule is believed to contribute to hematoma.12,13,20) However, satisfactory outcomes have been reported on hematoma removal without capsule removal and with careful follow-up that did not necessitate any surgical intervention.3,21) Endoscopic surgery may be effective for CEIH when malignancy can be ruled out preoperatively, especially if the risk of bleeding due to cirrhosis or other factors is high.21) In the present case, we conducted total resection because we initially suspected that the lesion was a malignant glioma. Despite reports of the efficacy of endoscopic or minimally invasive hematoma aspiration, craniotomy is the most employed method for CEIH removal.12) We believe that this fact represents the difficulty encountered in the preoperative diagnosis of CEIH and the ability to differentiate it from malignant brain tumors.
Progress and prognosisCEIH is generally recognized as a disease in which the symptoms of intracranial hypertension, such as headache and vomiting, progress over several weeks to months.1,6) Approximately 90% of patients with CEIH have a good prognosis with no sequelae.12) However, in cases such as the present one, in which the brain parenchyma is strongly compressed and exerts an edematous effect on the brainstem, the patient's condition may rapidly worsen in terms of impairment of consciousness. In such cases, emergency surgery should be promptly considered.
CEIH often progresses slowly and is associated with a favorable prognosis. However, in the case of severe cerebral edema and impairment of consciousness, emergency surgery should be considered. In cases of post-traumatic brain injury, including that sustained by surgery or radiotherapy, the possibility of CEIH should be considered, even if imaging studies are indicative of malignancy, such as malignant gliomas.
As the patient in this case had quit attending the hospital 9 years after therapy, we could only obtain images of the lesion upon admission; therefore, we could not track the progress of the disease. CEIH usually progresses gradually, and it had 11 years to do so in this case. However, we cannot rule out the possibility of a rapid growth of the hematoma and progression of the perifocal edema.
The patient consented to the submission of the case report to the journal.
The authors have no conflicts of interest to declare.