Abstract
Capillary hemangiomas are benign tumors comprising a lobulated proliferation of capillary vessels frequently located in the soft tissues of the neck and head. Spinal intradural capillary hemangiomas are rare, particularly intramedullary lesions. To our knowledge, only 31 cases of spinal intramedullary capillary hemangiomas have been reported. Here, we describe a rare case of a thoracic capillary hemangioma comprising extramedullary and intramedullary components. A 51-year-old male patient presented with bilateral lower extremity numbness and subsequent paraparesis, sensory disturbance, and bladder-bowel dysfunction with a subacute clinical course. Magnetic resonance imaging revealed a mass lesion with intramedullary and intradural extramedullary components at the Th9-10 vertebrae level and widespread spinal cord edema. Contrast-enhanced computed tomography revealed abnormal vessels on the dorsal spinal cord surface. Spinal angiography revealed a light-stained mass lesion fed by the radiculopial artery from the right Th11 intercostal artery. The tumor was resected en bloc, and the histological diagnosis was a capillary hemangioma. Postoperatively, the spinal cord edema diminished, and the patient was discharged from the convalescent rehabilitation ward. Although intramedullary capillary hemangioma is a rare spinal tumor and its preoperative diagnosis is difficult, it should be considered in the differential diagnosis of spinal intramedullary tumors.
Introduction
Capillary hemangioma is a benign tumor comprising a lobulated and alveolar proliferation of neoplastic capillary vessels frequently located in the soft tissues of the neck and head.1-3) Capillary hemangiomas involving the neuraxis are uncommon and are primarily intradural extramedullary lesions.3) Here, we describe a rare case of a thoracic capillary hemangioma comprising extramedullary and intramedullary components with widespread spinal cord edema leading to a subacute gait disturbance. Additionally, we reviewed the literature and discussed the characteristics of intramedullary capillary hemangiomas.
Case Report
A 51-year-old male patient presented with bilateral lower extremity numbness, starting spreading from the right sole two months before presentation. The patient also demonstrated consecutive gait disturbances and was admitted to our hospital. He could not walk independently. Left- and right-sided paralyses were observed in the lower limbs, and the manual muscle testing (MMT) results were as follows (right/left): hip flexors, 4/4; knee extensors, 3/3; ankle dorsiflexors, 4/4; extensor hallucis longus, 4/4; and ankle plantar flexors, 4/4. Decreased thermal and pain sensation of the trunk below the point 3 cm caudal to the umbilicus and bilateral lower extremities was observed. Touch sensation was also reduced on the bilateral lower extremities. In addition, decreased left-dominant deep sensitivity of the lower extremities was severe. Although the patient did not use a catheter, he experienced difficulty urinating and constipation. The modified McCormick scale4) was grade IV.
Magnetic resonance imaging (MRI) revealed a slightly high-intensity mass lesion on T2-weighted images, an iso-intensity lesion on T1-weighted images, and homogeneous enhancement at the level of the Th9-10 vertebrae (Fig. 1A-C). The MRI also showed widespread spinal cord edema extending from Th2 to the conus medullaris. Contrast-enhanced computed tomography revealed abnormal vessels running along the spinal cord's dorsal surface, suggesting a feeder or drainer for the lesion (Fig. 1D). The mass lesion had intramedullary and intradural extramedullary components and seemed to contact the dura mater (Fig. 1E-F). Spinal angiography revealed a mass lesion with light enhancement, fed by the radiculopial artery from the right Th11 intercostal artery (Fig. 1G-I). Preoperatively, the patient was diagnosed with a hemangioblastoma or meningioma, and tumor resection was performed.
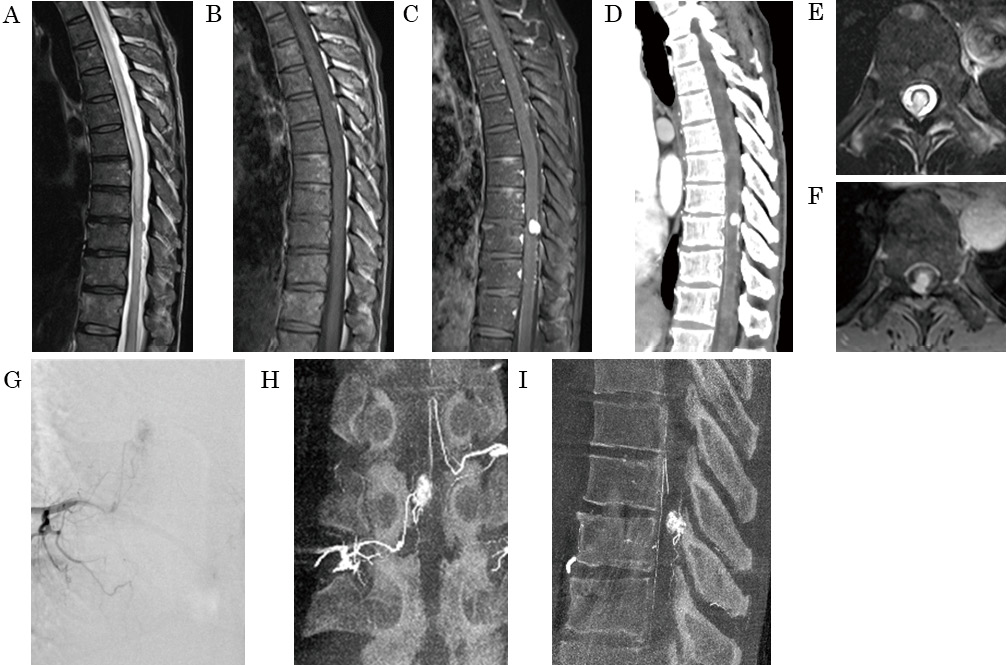
Bilateral partial laminectomy at Th8 and laminectomies at Th9-10 were performed. Following the dura incision, a hemangioma-like lesion extending from the extramedullary lesion to the intramedullary lesion and a dilated posterior spinal vein draining craniocaudally from the lesion was observed (Fig. 2A). Attachment to the dura mater was not observed. Intraoperative fluorescence imaging revealed poor blood flow associated with the tumor. After disconnection of the small vessels, which were considered feeders, the drainer was cut, and the tumor was resected en bloc (Fig. 2B-D). The pia mater on the dorsal surface of the extramedullary lesion was not observed. The continuity of the pia mater on the spinal cord was broken in the intramedullary component region. However, it was difficult to detect the border between the intramedullary and extramedullary components based on the pia mater and the tumor involvement. HE staining showed that the capillary vessels clustered and proliferated in a lobulated form (Fig. 3A-B). Immunostaining was positive for CD31 and CD34 and negative for EMA, D2-40, and STAT6 (Fig. 3C-D). The histological diagnosis was a capillary hemangioma.

Four months after the surgery, the patient was discharged from the convalescent rehabilitation ward. Motor paralysis was improved, and the MMT results were as follows (right/ left): hip flexors, 5/5; knee extensors, 5/5; ankle dorsiflexors, 4/4; long toe extensor, 4/4; and ankle plantar flexors, 4/4. He could walk with Lofstrand crutches. Although the impairment of pain, touch, and vibratory sensation partially remained, preoperative severe impairment of these sensations around the feet was relieved. Bowel and bladder problems were not observed, and the modified McCormick scale improved to grade III. Postoperative MRI showed improvement in spinal cord edema and no residual tumors (Fig. 3E-F).
Discussion
Capillary hemangiomas are benign tumors frequently located in the soft tissues of the neck and head.2,3) Among spinal lesions, this tumor is frequently located in the vertebral bodies.5,6) Although intradural lesions are rare, there are some reports of them. Tunthanathip classified spinal capillary hemangiomas into five: pediatric, epidural, intradural extramedullary, intramedullary, and hemangiomatosis.6) Among intradural lesions, most cases are extramedullary lesions; intramedullary lesions are extremely rare.6) In Japan, capillary hemangiomas account for 1.6% of intramedullary spinal tumors and are the seventh most common tumor.7) Therefore, it should be included in the differential diagnosis of spinal intramedullary tumors. However, the preoperative diagnosis of capillary hemangioma is difficult. Here, we describe a case of thoracic capillary hemangioma comprising extramedullary and intramedullary components and review spinal intramedullary capillary hemangiomas in detail.
Epidemiology
To our knowledge, only 31 cases of spinal intramedullary capillary hemangiomas have been reported (Table 1).1,3,6,8-21) Among them, approximately 19 cases included extramedullary and intramedullary components. Intradural capillary hemangiomas present in the fifth to sixth decades, with no sex predilection.2) Most intramedullary lesions occurred in middle-aged males (0-80 years old, median age = 56, male:female = 28:3).1,3,6,8-21) Cases in children are extremely rare.10,15,21) Cases of multiple lesions19) or intraneural cauda equina lesions22) have also been reported. Because a case of extramedullary capillary hemangioma with subpial growth has been reported previously, there may be more cases of mixed intra- and extramedullary capillary hemangiomas.23) In our case, although it was difficult to certify the tumor origin (e.g., the extramedullary or intramedullary side), the origin was considered the posterior surface of the spinal cord, as previously reported.1) Because the posterior spinal artery and vein were located in the subarachnoid but not the subpial space, tumors originating from the spinal cord's posterior surface could extend from the subarachnoid space. Intramedullary capillary hemangiomas could grow from the spinal cord surface, along with the extramedullary component, and pure intramedullary capillary hemangiomas are considered extremely rare.
Table 1
Literature review of intramedullary capillary hemangiomas
| First author |
Year |
Age (years),
Sex |
Involved level |
Location
(IM: Intramedullary,
EM: Extramedullary) |
Duration
(Y, years; M, months) |
Therapy |
Outcome |
| *until first operation VP shunt = ventriculoperitoneal shunt |
| Mawk |
1987 |
7 months, M |
Conus |
IM, EM, subcutaneous and cutaneous lesion |
3M |
resection (partial) |
improvement |
| Hida |
1993 |
50, M |
C3-T1 |
IM |
3M * |
biopsy |
no change |
| Rancaroli |
2000 |
42, F |
T11 |
IM (no detail) |
1.5Y |
resection |
recovery |
| Rancaroli |
2000 |
50, M |
T11 |
IM (no detail) |
1Y |
resection and radiotherapy |
little improvement |
| Rancaroli |
2000 |
53, M |
Conus |
IM (no detail) |
2Y |
resection |
leg weakness |
| Rancaroli |
2000 |
64, M |
T10 |
IM (no detail) |
2Y |
resection |
recovery |
| Shin |
2000 |
66, F |
T8-9 |
IM, EM |
8M |
resection (incomplete) |
improvement |
| Rancaroli |
2000 |
74, M |
Multiple |
pial surface |
9M |
resection (two of multiple lesions) |
no change |
| Andaluz |
2002 |
41, M |
T12 |
IM, EM |
3M |
resection |
recovery |
| Abe |
2004 |
65, M |
T5 |
IM |
2M |
resection |
little improvement |
| Abe |
2004 |
43, M |
T7 |
IM, EM |
2M |
resection |
recovery |
| Abe |
2004 |
51, M |
T11 |
IM, EM |
1M |
resection |
recovery |
| Abe |
2004 |
64, M |
T7 |
IM, EM |
2M |
resection |
recovery |
| Abe |
2004 |
71, M |
T11 |
IM, EM |
3M |
resection |
little improvement |
| Abe |
2004 |
80, M |
T9 |
IM, EM |
4M |
resection |
worsening |
| Iannelli |
2005 |
3 months, M |
T4-7 |
IM, EM |
2-3M |
VP shunt and resection |
improvement |
| Kelleher |
2005 |
57, M |
T9-10 |
IM, EM |
7M |
resection |
improvement |
| Kasukurthi |
2009 |
47, M |
T3 |
IM, EM |
Several months |
resection |
recovery |
| Wu |
2013 |
49, M |
T1-2 |
IM |
2Y |
resection |
improvement |
| Wu |
2013 |
63, F |
T11 |
IM |
2M |
resection |
improvement |
| Wu |
2013 |
18, M |
T7-8 |
IM |
5M |
resection |
improvement |
| Wu |
2013 |
47, M |
C7-T1 |
IM |
14M |
resection |
improvement |
| Wu |
2013 |
59, M |
T3-4 |
IM, EM |
1Y |
resection (recurrence after subtotal resection) |
improvement |
| Gonzalez |
2014 |
59, M |
T7-8 |
IM, EM |
7M |
resection (incomplete) |
no complication |
| Tunthanathip |
2017 |
31, M |
C5-6 |
IM, multiple cervicothoracic subpial lesions |
1M |
resection |
improvement |
| Forbes |
2018 |
75, M |
C4 |
IM, EM |
6M |
resection |
recovery |
| Vaishya |
2019 |
2 months, M |
T10-12 |
IM, EM |
2M |
VP shunt and resection |
improvement |
| Zhao |
2022 |
56, M |
T9-10 |
IM, EM |
1M |
resection |
recovery |
| Zhao |
2022 |
56, M |
T8 |
IM, EM |
5M |
resection |
improvement |
| Zhao |
2022 |
60, M |
L1 |
IM, EM |
4M |
resection |
improvement |
| Protas |
2023 |
63, M |
T8-9 |
IM, EM |
0.5M |
resection |
improvement |
Capillary hemangiomas have slow growth potential and can cause slowly progressive symptoms.2) The appearance of any of the symptoms of back pain, lower limb weakness, and lower limb numbness are reportedly associated with intramedullary capillary hemangiomas.1,3,6,8,9,12-14,16-20) Some patients with intramedullary capillary hemangiomas reported that the duration of illness was several years,18) whereas others reported that it was several months1) (Table 1). Along with severe spinal cord edema, intramedullary capillary hemangiomas can cause progressive neurological symptoms, as in our case.1,12) To our knowledge, sudden onset bleeding due to the intramedullary lesion has not been reported.3) However, a case of an extramedullary lesion with bleeding has been reported.24)
Radiological studies
Shin et al. reported the MRI findings of intradural capillary hemangiomas, especially of the extramedullary and intramedullary components.17) The tumor shows iso-intensity with the spinal cord on T1-weighted images, hyperintensity relative to the spinal cord on T2-weighted images, and strong homogeneous enhancement.17) Because intramedullary capillary hemangiomas could involve extramedullary component, T2-weighed images are useful for differentiating tumors from intradural extramedullary tumors, such as meningiomas, which show iso-intensity or low-intensity on T2-weighted image.17) Gonzalez et al. reported an intra and extramedullary capillary hemangioma with flow voids on the dorsal spinal cord surface and severe spinal cord edema.12) Flow voids could be a characteristic finding of capillary hemangiomas and hypervascular tumors, such as hemangioblastomas. Although preoperative diagnosis of spinal intramedullary capillary hemangiomas is difficult, they should be considered in the differential diagnosis of hypervascular intramedullary tumors with extramedullary components.
Few studies have reported spinal angiography findings. Abe et al. reported two cases of spinal capillary hemangiomas; one of them was an intra and extramedullary lesion, which showed a hypervascular mass fed by the radicular artery.1) In our case, spinal angiography showed that the radiculopial artery was the feeding artery of the lesion.
Pathology
Capillary hemangioma is a benign tumor comprising a lobular architecture of capillary vessels.1) This architecture helps distinguish capillary hemangiomas from other vascular diseases.1) For example, it must be distinguished from cavernous hemangiomas, capillary telangiectasias, hemangioblastomas, and solitary fibrous tumors.1) Each disease differs from capillary hemangioma in the following characteristics: cavernous hemangiomas comprising dilated hyaline vessels with thrombosis, periventricular hemosiderin deposition, and calcification.1) Capillary telangiectasias have a neural parenchyma between the vessels.1) Hemangioblastomas contain foamy stromal cells.1) Solitary fibrous tumors have a stag-horn vasculature.1) Immunohistochemistry is also helpful for the diagnosis. Capillary hemangiomas are positive for CD31, a vascular endothelial cell marker, and negative for D2-40.12) In addition, it is negative for S100, inhibin, and neuron-specific enolase, which is helpful for hemangioblastoma exclusion.12) Vascular endothelial growth factor (VEGF) induces an angiogenic response and has also been implicated in tumor angiogenesis.25) Capillary hemangiomas are positive for VEGF in the proliferating endothelial cells without vessel lumen formation.1)
Treatment
Early microsurgery should be performed to treat symptomatic intramedullary capillary hemangiomas.3) Most lesions do not adhere to the spinal cord, and complete resection is desired.1,3) If complete resection is difficult, partial removal is acceptable with adequate bleeding control and follow-up.3) Because VEGF has been reported as an autocrine factor in capillary hemangiomas, which increases vascular permeability, leading to edema, primary lesion removal might induce tumor regression.1) However, one case of recurrence after subtotal resection was reported3) (Table 1). Although temporary aggravation of neurological symptoms can occur, especially deep sensations when resecting the lesion of the posterior spinal cord surface, symptoms improved after surgery in many cases reported18) (Table 1). Systemic administration of corticosteroids and interferons could effectively treat brain lesions.1) Recently, the effectivity of steroid administration and radiation therapy for a case of an extramedullary spinal lesion has also been reported.23)
Conclusion
Intramedullary capillary hemangiomas can be diagnosed via histopathological examination. This report describes intramedullary capillary hemangioma characteristics in terms of epidemiology, clinical course, and radiological findings. Although intramedullary capillary hemangiomas are rare spinal tumors and the preoperative diagnosis is difficult, it should be considered in the differential diagnosis of spinal intramedullary tumors.
Data Availability Statement
The authors declare that data pertaining to this case report will be made available upon request.
Author Contribution Statement
YY and HS were responsible for writing the report, conducting the literature search, extracting and analyzing the data, interpreting the results, and updating the reference lists. FH, TS, KA, RS, TM, and MA were responsible for extracting and analyzing the data and interpreting the results. HY and YY provided feedback regarding the reports.
Funding
No financial assistance was obtained for this study.
Ethical Approval
For this case report, informed consent was obtained from the patient for publication and an institutional ethical review was deemed unnecessary.
Conflicts of Interest Disclosure
The authors declare no conflicts of interest relevant to this study.
References
- 1) Abe M, Tabuchi K, Tanaka S, et al.: Capillary hemangioma of the central nervous system. J Neurosurg 101: 73-81, 2004
- 2) Nowak DA, Widenka DC: Spinal intradural capillary haemangioma: a review. Eur Spine J 10: 464-472, 2001
- 3) Wu L, Deng X, Yang C, Xu Y: Intramedullary spinal capillary hemangiomas: clinical features and surgical outcomes: clinical article. J Neurosurg Spine 19: 477-484, 2013
- 4) Manzano G, Green BA, Vanni S, Levi AD: Contemporary management of adult intramedullary spinal tumors-pathology and neurological outcomes related to surgical resection. Spinal Cord 46: 540-546, 2008
- 5) Pastushyn AI, Slin'ko EI, Mirzoyeva GM: Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol 50: 535-547, 1998
- 6) Tunthanathip T, Rattanalert S, Oearsakul T, Kanjanapradit K: Spinal capillary hemangiomas: two cases reports and review of the literature. Asian J Neurosurg 12: 556-562, 2017
- 7) Endo T, Inoue T, Mizuno M, et al.: Current trends in the surgical management of intramedullary tumors: A multicenter study of 1,033 patients by the neurospinal society of Japan. Neurospine 19: 441-452, 2022
- 8) Protas M, Ojukwu DI, Draytsel DY, Galgano MA: Illustrative resection of mixed intra- and extramedullary thoracic spinal cord capillary hemangioma. Surg Neurol Int 14: 226, 2023
- 9) Zhao Z, Zheng J, Zhou Y: Intradural extramedullary capillary hemangioma with intramedullary component: A case series. Medicine (Baltimore) 101: e29862, 2022
- 10) Vaishya S, Chauhan A, Patir R, Gupta RK: Intramedullary capillary hemangioma presenting with hydrocephalus and spastic paraparesis in 2-month-old infant. World Neurosurg 125: 451-455, 2019
- 11) Forbes JA, Teschan N, Jones SH, Parry P, Simonet L, Swamy NK: Cervical corpectomy for resection of ventral intramedullary capillary hemangioma with circumferential involvement of the anterior spinal artery: case report. J Neurosurg Spine 29: 144-149, 2018
- 12) Gonzalez R, Spears J, Bharatha A, Munoz DG: Spinal lobular capillary hemangioma with an intramedullary component. Clin Neuropathol 33: 38-41, 2014
- 13) Kasukurthi R, Ray WZ, Blackburn SL, Lusis EA, Santiago P: Intramedullary capillary hemangioma of the thoracic spine: case report and review of the literature. Rare Tumors 1: e10, 2009
- 14) Kelleher T, Aquilina K, Keohane C, O'Sullivan MG: Intramedullary capillary haemangioma. Br J Neurosurg 19: 345-348, 2005
- 15) Iannelli A, Lupi G, Castagna M, Valleriani A, Becherini F: Intramedullary capillary hemangioma associated with hydrocephalus in an infant. J Neurosurg 103: 272-276, 2005
- 16) Andaluz N, Balko MG, Stanek J, Morgan C, Schwetschenau PR: Lobular capillary hemangioma of the spinal cord: case report and review of the literature. J Neurooncol 56: 261-264, 2002
- 17) Shin JH, Lee HK, Jeon SR, Park SH: Spinal intradural capillary hemangioma: MR findings. AJNR Am J Neuroradiol 21: 954-956, 2000
- 18) Roncaroli F, Scheithauer BW, Krauss WE: Capillary hemangioma of the spinal cord. Report of four cases. J Neurosurg 93: 148-151, 2000
- 19) Roncaroli F, Scheithauer BW, Deen HG Jr: Multiple hemangiomas (hemangiomatosis) of the cauda equina and spinal cord. Case report. J Neurosurg 92: 229-232, 2000
- 20) Hida K, Tada M, Iwasaki Y, Abe H: Intramedullary disseminated capillary hemangioma with localized spinal cord swelling: case report. Neurosurgery 33: 1099-1101, 1993
- 21) Mawk JR, Leibrock LG, McComb RD, Trembath EJ: Metameric capillary hemangioma producing complete myelographic block in an infant. Case report. J Neurosurg 67: 456-459, 1987
- 22) Nowak DA, Gumprecht H, Stölzle A, Lumenta CB: Intraneural growth of a capillary haemangioma of the cauda equina. Acta Neurochir (Wien) 142: 463-467; discussion 467-468, 2000
- 23) Matsumoto H, Shimokawa N, Sato H, Takami T: Adjuvant multimodal treatment for spinal intradural extramedullary capillary hemangioma with subpial growth: illustrative case. J Neurosurg Case Lessons 5: CASE2314, 2023
- 24) Panero I, Eiriz C, Lagares A, Toldos O, Panero A, Paredes I: Intradural-extramedullary capillary hemangioma with acute bleeding: case report and literature review. World Neurosurg 108: 988.e7-988.e14, 2017
- 25) Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 9: 669-676, 2003



