2024 Volume 11 Pages 135-140
2024 Volume 11 Pages 135-140
Stroke-like migraine attacks after radiation therapy (SMART) syndrome, a delayed sequela of cranial radiotherapy encountered rarely, occurs due to transient neurological deficits coupled with migraine episodes. This case report describes an occurrence of SMART syndrome in an individual 8 years after receiving medulloblastoma treatment. The subject, a 21-year-old male, experienced abrupt aphasia and right-sided hemiparesis. Arterial spin labeling (ASL) revealed initial cerebral hypoperfusion in the left temporal and parietal regions, with no tumor resurgence or notable ischemic alterations. Two days later, the symptoms disappeared completely; nevertheless, at that time, ASL presented cerebral hyperperfusion in the same lobule. The subject experienced a pulsating headache and nausea the next day. In the context of SMART syndrome, this fluctuation in cerebral blood flow indicated by ASL is a unique finding. The significance of this case lies in the documentation of the dynamic evolution of cerebral perfusion in SMART syndrome via ASL, thereby elucidating its underlying pathophysiology. As hemiplegic migraine shows a similar cerebral perfusion pattern to SMART syndrome, we inferred an unexplored but shared pathophysiology among hemiplegic migraine and SMART syndrome. Through this successful capture of these distinct cerebral blood flow alterations, from hypoperfusion to hyperperfusion, our understanding of the pathophysiological intricacies inherent to SMART syndrome will be enhanced.
Stroke-like migraine attacks after radiation therapy (SMART) syndrome represents an infrequent but significant delayed complication of cranial radiotherapy, which is characterized by transient focal neurological deficits and migraine episodes that emerge years after the initial treatment.1,2) Although the clinical presentation is distinctive, accurately diagnosing SMART syndrome is still a daunting challenge, and the underlying pathophysiological mechanisms remain poorly understood.3,4) Reports on cerebral blood flow (CBF) dynamics that reported a transient elevation in CBF several days following symptom onset are few.2,4) Nevertheless, one report captured a decrease in CBF immediately after onset.5) Additionally, 14%-18% of patients diagnosed with SMART syndrome subsequently develop cerebral infarction, which indicates a potential ischemic component to the condition.2) Therefore, no settled view on CBF changes in SMART syndrome remains.
In this paper, a case of SMART syndrome that manifested 8 years after the patient received treatment for medulloblastoma is presented. In this case, the significant alterations in CBF were notable, specifically the transition from initial cerebral hypoperfusion to later hyperperfusion. Using arterial spin labeling (ASL), we meticulously monitored and documented these changes, providing valuable insights into the cerebrovascular dynamics associated with this rare syndrome.
At our institution, a 13-year-old male presented with a headache. Using magnetic resonance imaging (MRI), a faint, heterogeneously contrast-enhanced tumor within the fourth ventricle was identified, exerting pressure on the brainstem (Fig. 1A). The patient underwent tumor resection, and the pathology confirmed classic medulloblastoma. Postoperatively, the treatment protocol included chemotherapy and craniospinal irradiation (24 Gray [Gy] over 16 sessions) and localized irradiation (27.2 Gy over 17 sessions).

Pre-onset status of the patient with SMART Syndrome.
(A) A preoperative contrast-enhanced MRI-T1WI displays a mass within the fourth ventricle (white arrow) in a sagittal view. Panels B and C are postoperative follow-up MRIs, contrast-enhanced T1WI, and acquired 8 years after the surgical intervention. Panel B presents a sagittal view of the cervical and thoracic spinal cord, demonstrating a residual seeding lesion whose size has remained unchanged (white arrowheads). Panel C offers a sagittal view of the cranium.
MRI, magnetic resonance imaging; SMART, stroke-like migraine attacks after radiation therapy; T1WI, T1-weighted image
Subsequent imaging showed disseminated lesions on the cerebellar surface, the Sylvian fissure, and the spinal cord, requiring spinal tumor resections 3 and 7 years post-initial diagnosis. Despite these interventions, a thin disseminated lesion persisted. Consequently, maintenance chemotherapy with temozolomide, etoposide, and cyclophosphamide was initiated, effectively controlling disease progression (Fig. 1B). Notably, during this period, no tumor recurrence was observed in the primary surgical cavity (Fig. 1C). The patient's condition remained stable under ongoing chemotherapy, 8 years post-diagnosis. He exhibited limited mobility in both lower limbs but maintained a largely independent lifestyle. At the age of 21, he presented to our hospital with sudden onset hemiplegia and aphasia.
This incident marked the patient's first experience with aphasia and hemiplegia. Upon examination, he was conscious, with a Glasgow coma scale (GCS) score of E4V4M6, but demonstrated motor aphasia and right-sided paralysis. Through MRI diffusion-weighted imaging (DWI), no significant ischemic changes was observed (Fig. 2A and B). By contrast, via magnetic resonance angiography (MRA), obscurity in the left M3 segment of the middle cerebral artery (MCA) was found (Fig. 2C). ASL revealed reduced perfusion in the left temporal and parietal lobes (Fig. 3A). Using these findings, we determined that the patient had incomplete ischemia and initiated aspirin.

MRI progress during the first hospitalization.
Panels A, B, and C are MRIs acquired upon initial presentation of aphasia and right-sided hemiplegia. Panel A and B are DWI scans. Panel C is an MRA image showing compromised visualization of the left middle cerebral artery beyond the M3 segment (white arrow). Panels D, E, and F represent cranial MRIs obtained on the third day of hospitalization. Panel D is an MRA image. Panels E and F are contrast-enhanced MRI T1WI, revealing no tumor recurrence (arrowhead) evidence. Panels G and H are cranial CT scans conducted on the fourth day.
R and L indicate right and left, respectively.
CT, computed tomography; DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; T1WI, T1-weighted image
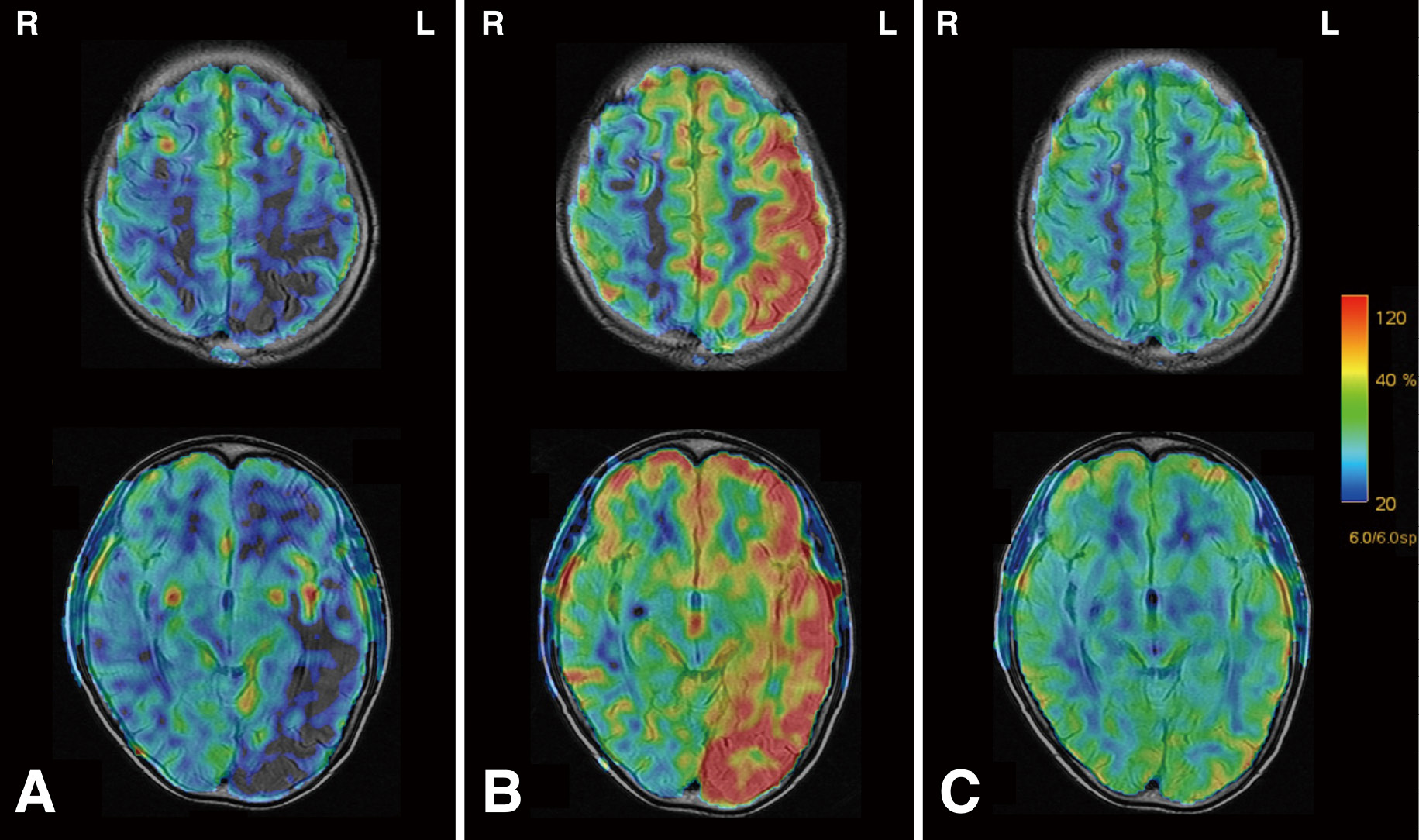
Progress of ASL images.
The progression of cerebral perfusion is depicted. The color bar on the right represents CBF, with red indicating hyperperfusion and blue indicating low perfusion. Panel A is acquired at the time of hospital admission. Panel B is on the third day after hospitalization. Panel C is captured on the 15th-day post-onset, after discharge.
R and L indicate right and left, respectively.
ASL, arterial spin labeling; CBF, cerebral blood flow
On the second day, the patient's condition deteriorated, evidenced by aggravated aphasia, loss of coherent speech, and somnolence (GCS: E3V3M5). Electroencephalography revealed decreased activity in the left cerebral hemisphere, as shown by hemispheric slow waves, with no signs of epileptiform activity. Cerebrospinal fluid analysis depicted elevated protein and cell counts, which suggested meningeal dissemination. Polymerase chain reaction assays for various viruses produced negative results. In the absence of fever or blood abnormalities, encephalitis was deemed unlikely.
By the third day, the patient's aphasia and hemiparesis had notably improved, although a mild headache persisted. Follow-up MRA presented significant improvement in the left MCA compared to the admission state, indicating hyperperfusion (Fig. 2D). Surprisingly, ASL also demonstrated left hemispheric hyperperfusion (Fig. 3B). Contrast-enhanced MRI showed no apparent areas of contrast in the extraction cavity or cortex and no evident tumor recurrence or progression (Fig. 2E and F).
On the fourth day, the patient developed a pulsating headache and nausea, predominantly affecting the left temporal region. Computed tomography (CT) imaging revealed sulci obscuration in the left hemisphere, particularly in the left parietal-temporal lobule, suggesting brain edema (Fig. 2G and H). We interpreted this CT imaging as cerebral swelling secondary to hyperperfusion. Consequently, we discontinued aspirin and administered glycerin to alleviate intracranial pressure. The patient's headache gradually improved, and on the sixth day, he was discharged, with a residual mild headache. On the 15th-day post-onset, MRI confirmed homogenous perfusion across both cerebral hemispheres, without sequelae (Fig. 3C). Considering the history of radiation therapy, reversible neurological symptoms, migraine-like headaches, and the excluding of tumor recurrence, cerebral infarction, seizure, and encephalitis, we diagnosed SMART syndrome.
Eight months later, the patient returned with symptoms of left hemiplegia, left hemispatial neglect, and altered consciousness (GCS: E3V4M6). DWI displayed no notable high signal intensity areas, and MRA indicated right MCA hypoperfusion (Fig. 4A and B). Considering these findings, we suspected a recurrence of the SMART syndrome and managed him with supplemental fluids and without antiplatelet therapy. Over the subsequent days, his neurological symptoms spontaneously resolved, although a persistent headache remained. Follow-up MRA showed restored CBF (Fig. 4C).

MRI acquired after the patient's second hospital admission.
Panel A displays DWI-MRI, and panel B presents an MRA image, illustrating the suboptimal delineation of the right middle cerebral artery (white arrow). Panel C is a follow-up MRA image acquired post-discharge.
R and L indicate right and left, respectively.
DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging
We report a case of SMART syndrome, manifesting 8 years post-medulloblastoma therapy. Notably, to demonstrate dynamic changes in CBF in SMART syndrome, ASL imaging was utilized. Initially, the patient presented with focal neurological symptoms such as paralysis and aphasia with hypoperfusion of the implicated lesion. Subsequently, the lesion exhibited hyperperfusion, coinciding with the remission of neurological symptoms, leaving the patient with migraine-like headaches.
SMART syndrome, first identified by Shuper et al. in 1995, is a rare, delayed complication of cranial irradiation.1) This condition, characterized by migraine-like headaches and transient focal neurological deficits originating at the irradiated site, manifests years after intracranial malignancy radiation therapy.1-3) Reports of SMART syndrome are scarce, with fewer than 50 cases documented since its initial description.4) The syndrome can present in individuals aged 3.5-88, with a median latency of approximately 14 years from irradiation to onset.6) Symptoms are transient and localized to specific cortical areas, yet approximately 14%-18% of patients suffer strokes with lasting effects, and 55%-62% experience recurrent episodes.2) Our case, like numerous other reports, had recurrent attacks, but none of the attacks developed into a cerebral infarction.
In terms of radiation dose, the initial reports on SMART syndrome suggested that the onset of the syndrome might require a minimum dose of 50 Gy.1) Nevertheless, subsequent findings have reported occurrences of SMART syndrome at significantly lower doses, which were as low as 15 Gy, leading to the current understanding that the radiation dose may not be a critical factor in the development of SMART syndrome.3,6) Moreover, instances of whole-brain irradiation for posterior fossa tumors have been associated with attacks originating in the cerebral hemisphere.7) In this case, he underwent 24 Gy of whole-brain irradiation, which we inferred as the cause of triggering two separate attacks, each emanating from the left and right cerebral hemispheres, respectively.
Diagnostic criteria for SMART syndrome, as first established by Black et al. in 2006, include the following: A) remote history of external beam cranial irradiation without evidence of residual or recurrent neoplasm; B) prolonged, reversible signs and symptoms referable to a unilateral cortical region beginning years after irradiation; C) transient, diffuse, unilateral cortical gadolinium enhancement of the cerebral gyri sparing the white matter within a previous radiation field; and D) not attributed to another disorder.8) In our case, three out of four points were concordant. Despite the absence of gadolinium enhancement, recent literature suggests that such changes are not mandatory for diagnosis.2,3) Diagnosis hinges on imaging features indicative of unilateral gyriform enhancement and cortical swelling, and other case reports have captured the same findings using fluid-attenuated inversion recovery images.2,3) The change is supposed to occur within a week of onset.3) Our patient's CT imaging, conducted 4 days post-onset, revealed corticomedullary boundary obscuration and cerebral sulcus blurring in the left hemisphere, consistent with gyrus swelling. Alongside a history of extensive irradiation, recurrent migraine-like headaches, transient focal neurological symptoms, and the absence of neoplastic recurrence or other conditions, this case culminated in a SMART syndrome diagnosis.
Our case is notable for its detailed documentation of the dynamic changes in cerebral blood perfusion associated with SMART syndrome. Several case reports and reviews have described an increase in CBF, whereas one report noted a decrease in CBF immediately following the onset.2,4,5) Consequently, no consensus regarding CBF in SMART syndrome exists. Our ASL imaging observations indicate an initial reduction in CBF, followed by a transition to hyperperfusion. This progression pattern, which aligns with each of the previously reported cases on CBF changes, may assist in differentiating SMART syndrome from ischemic conditions such as reversible cerebral vasoconstriction syndrome and hyperexcitability states such as epileptic seizures.2,9) Furthermore, it provides valuable insights into the pathophysiology of the syndrome.
The etiology of SMART syndrome is hypothesized to involve delayed cerebral irradiation injuries, including white matter necrosis, vascular endothelial damage, demyelination, and gliosis.2,10) However, its precise pathogenesis remains largely elusive.3,4) The syndrome's symptomatology and its association with migraine attacks parallel hemiplegic migraine, as defined in the International Classification of Headache Disorders, 3rd Edition, characterized by migraine with aura and motor paralysis.4,11) Both conditions exhibit reversible, short-lived focal neurological symptoms.
Interestingly, migraine headaches typically cause a decrease in blood flow during the attack, which is followed by an increase.12,13) Recent studies have documented similar CBF patterns in hemiplegic migraine, initially presenting as hypoperfusion and later transitioning to hyperperfusion.14-16) This parallel suggests a potential shared pathophysiological mechanism between SMART syndrome and hemiplegic migraine.
The link between migraines and mitochondrial dysfunction is well-established, with patients diagnosed with familial hemiplegic migraine presenting mitochondrial morphological abnormalities.17,18) Mitochondrial diseases are also associated with migraine-like headache episodes, and pharmacological agents enhancing mitochondrial metabolism have been effective in alleviating migraines.17) Ota et al. have proposed radiation therapy-induced mitochondrial dysfunction as a potential mechanism underlying SMART syndrome.2) Ionizing radiation and subsequent free radical production can impair mitochondrial function, potentially leading to neuronal hyperexcitability, cortical spreading depression, and migraine headache episodes.
Thus, exploring the parallels between SMART syndrome and hemiplegic migraine could provide valuable insights into their shared pathophysiological mechanisms. Although the prevalence of hemiplegic migraine per population in Denmark at the end of 1999 was estimated to be approximately 0.01%,19) we could not find the prevalence of SMART syndrome based on previous studies. Nevertheless, we infer that the prevalence of both conditions is rare. SMART syndrome is generally characterized by altered imaging findings centered on the irradiation site and symptoms originating in the same region.1-3) We speculate that SMART syndrome may have obtained a localized predisposition to hemiplegic migraine due to radiation-induced mitochondrial dysfunction and other effects. However, numerous aspects of the pathogenesis remain unclear, including why such symptoms first appear many years after irradiation.
Furthermore, in SMART syndrome after whole-brain irradiation, including our case, all intracranial areas can be the origin of symptoms. In our case, each of the left and right cerebral hemispheres was involved in a separate attack, which is indistinguishable from a simple complication of hemiplegic migraine. Thus, the relationship between SMART syndrome and hemiplegic migraine awaits further case series.
There are several limitations to this report. Mainly, it is based on a singular case study, requiring additional cases to validate the specificity of the observed perfusion pattern in SMART syndrome. Furthermore, the patient was undergoing maintenance chemotherapy and receiving temozolomide, etoposide, and cyclophosphamide, concurrent with the emergence of neurological symptoms. Compounding this, residual, albeit controlled, lesions persisted in the spinal cord and cerebral surface. Although residual lesions or maintenance chemotherapy may affect the dynamic cerebral flow changes, the scarcity of prior reports detailing a clinical trajectory like our report results in the association between these factors and the observed symptoms being tentative. However, we cannot ignore the influence of residual lesions and maintenance chemotherapy.
To conclude, this case of SMART syndrome uniquely shows a transition from initial cerebral hypoperfusion to subsequent hyperperfusion, as depicted by ASL. This distinctive perfusion pattern could not only facilitate the diagnosis of SMART syndrome but also reflect similar changes observed in hemiplegic migraine, suggesting a common underlying pathophysiology. Continued investigation of this correlation will enhance our understanding and management of these complex neurological conditions.
Since not all authors were native English speakers, we employed ChatGPT 3.5 to review the manuscript grammatically. We would like to express our appreciation to a native speaker for reviewing our manuscript for grammatical accuracy.
ASL, arterial spin labeling; CBF, cerebral blood flow; CT, computed tomography; DWI, diffusion-weighted imaging; GCS, Glasgow coma scale; Gy, Gray; MCA, middle cerebral artery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; SMART, stroke-like migraine attacks after radiation therapy; T1WI, T1-weighted image.
The subject (he) provided written informed consent to publish the information and images about his experience.
The authors declare no conflict of interest.