2016 Volume 3 Issue 4 Pages 111-114
2016 Volume 3 Issue 4 Pages 111-114
Spinal intradural extramedullary inflammatory pseudotumor (IPT) is an extremely rare entity. Spontaneous shrinking of a spinal IPT has never been reported. A case of an IPT of the cauda equina that regressed spontaneously is presented. A 78-year-old woman presented with hypoesthesia of both lower legs in the L4 nerve root distribution and motor weakness of the right leg. Preoperative CT myelography and MRI showed two tumor-like lesions located at T12-L1 and L2-3. The lesion at the T12-L1 level appeared to encase several nerve roots. The preoperative diagnosis was ependymoma, schwannoma, or malignant lymphoma. The tumors were biopsied. In the operation, the lesion turned out to consist of swollen and adherent nerve roots. On histopathological examination of the biopsied nerve roots, they were diagnosed as IPT. The patient’s symptoms improved gradually without any treatment after the operation. The IPTs regressed on the postoperative MR images and disappeared at one year. This is the first report of spontaneous regression of an IPT in the spinal region. IPT should be considered in the differential diagnosis of a tumor that appears to involve several nerve roots on preoperative imaging, but surgery is necessary for diagnosis. Complete resection is not absolutely required if an intraoperative pathological diagnosis of the frozen section reveals IPT.
Inflammatory pseudotumor (IPT) is a histologically benign, ubiquitous lesion of unknown cause. IPT is defined as an inflammatory process that includes a diverse group of lesions characterized by inflammatory cell infiltration and variable necrotic locations.1) IPT has been described using various terms, such as “plasma cell granuloma” or “inflammatory myofibroblastic tumor”.2) IPT usually occurs in the lung and the orbit,3) but rarely in the central nervous system, and spinal intradural extramedullary lesions are extremely rare. To the best of our knowledge, only ten cases have been reported.2,4–12) The tumors were usually located on the spinal cord meninges2,4–12) and treated by surgical resection. The first case of an IPT located in the cauda equina, which shrank spontaneously without any treatment, is presented.
A 78-year-old woman developed severe pain and numbness in her bilateral buttocks and legs at the end of March 2011. The pain was triggered by coughing. A week later, she developed weakness of her right leg. The monoplegia in her right leg progressed very quickly, and she became unable to walk. She was referred to our hospital at the beginning of April. Before the onset of symptoms, she had been in good general health. Physical examination revealed hypoesthesia of both lower legs in the L4 nerve root distribution and motor weakness of the right leg on manual muscle testing: iliopsoas muscle 2/5, quadriceps muscle 2/5, tibialis anterior muscle 2/5, and gastrocnemius muscle 2/5. Magnetic resonance imaging (MRI) demonstrated two mass lesions at the T12-L1 and L2-3 levels (Fig. 1), which were not detected on MR images taken at the other hospital one year earlier. These lesions were low intensity on T1-weighted images, high intensity on T2-weighted images, and homogeneously enhanced with gadolinium administration (Fig. 1A–C). No other lesions were observed in her brain or other spinal areas. Myelography demonstrated that the lesion at T12-L1 moved cranially and caudally with changes in the patient’s position (Fig. 1D, E). CT myelography showed a tumor-like lesion, resembling a jellyfish that appeared to involve several nerve roots at T12-L1 (Fig. 1F). Blood examinations showed no inflammatory changes, and a chest X-ray showed no abnormalities. The preoperative differential diagnosis was ependymoma, schwannoma, or malignant lymphoma.

Preoperative magnetic resonance imaging (MRI) shows two lesion masses, one occupying nearly the entire thecal sac from the T12 to the L1 level, and the other at the dorsal side in the thecal sac from the L2 to the L3 level. (A) These masses are low intensity on T1WI, (B) high intensity on T2WI, (C) homogeneously enhanced with gadolinium administration. (D, E) Myelography shows that the tumor at T12-L1 moves cranially and caudally with changes in the patient’s position (*: T12 level). (F) CT myelography shows branching of several nerve roots from the tumor at T12-L1.
Surgical removal of the lesions was attempted in the middle of April. Laminectomy was performed from the T12 to L3 laminae, and the locations of the tumor-like lesion were confirmed by ultrasonography before opening the thecal sac. Incision of the dura mater was performed at the T12-L1 level to sample the diseased tissue for pathological diagnosis during surgery. The nerve roots were swollen in this area and adhered tightly to each other (Fig. 2). The nerve roots were dissected, but the tumor could not be found. Finally, it was concluded that the tumor-like lesion seen on MRI and ultrasonography was indeed a pseudotumor that consisted of thickened nerve roots that adhered to each other. Sensory nerve roots that did not evoke muscle contraction of the legs or buttocks were identified with electronic stimulation, and one of the nerve roots was sampled. The intraoperative pathological examination of the frozen section showed inflammatory change without neoplastic changes. Sampling of two other nerve roots was performed, but the results of the intraoperative pathological examinations were the same. Thus, the operation finished, and it was decided to select methods of treatment according to the postoperative histopathological diagnosis.

Intraoperative photograph shows that most of the cauda equina nerve roots are swollen in this area and adhere tightly to each other (asterisk). No other intradural or extradural masses are detected.
The lesion was characterized by polymorphous infiltration consisting of numerous mature plasma cells, plasmacytoid cells, large basophilic transformed lymphocytes (immunoblasts), and small to medium-sized lymphocytes. Some of the immunoblasts had large vesicular nuclei and prominent nucleoli resembling Hodgkin cells (Fig. 3A). Moreover, the lesion contained scattered spindle-shaped cells (Fig. 3A). Staining with CD20 (Fig. 3B) and CD3 (Fig. 3C) showed the mixed nature of the small and medium lymphocytes. The majority of immunoblasts showed the B-cell phenotype (Fig. 3C). Immunohistochemical studies of light chain determinants for plasma cells, plasmacytoid cells, and B-immunoblasts demonstrated a polyclonal pattern (Fig. 3D, E). The majority of the spindle-shaped cells were positive for vimentin (Fig. 3F), with a portion positive for muscle-specific actin (Fig. 3G) and smooth muscle actin, but negative for CD246. Only a few IgG4-positive plasma cells were observed in the lesion.
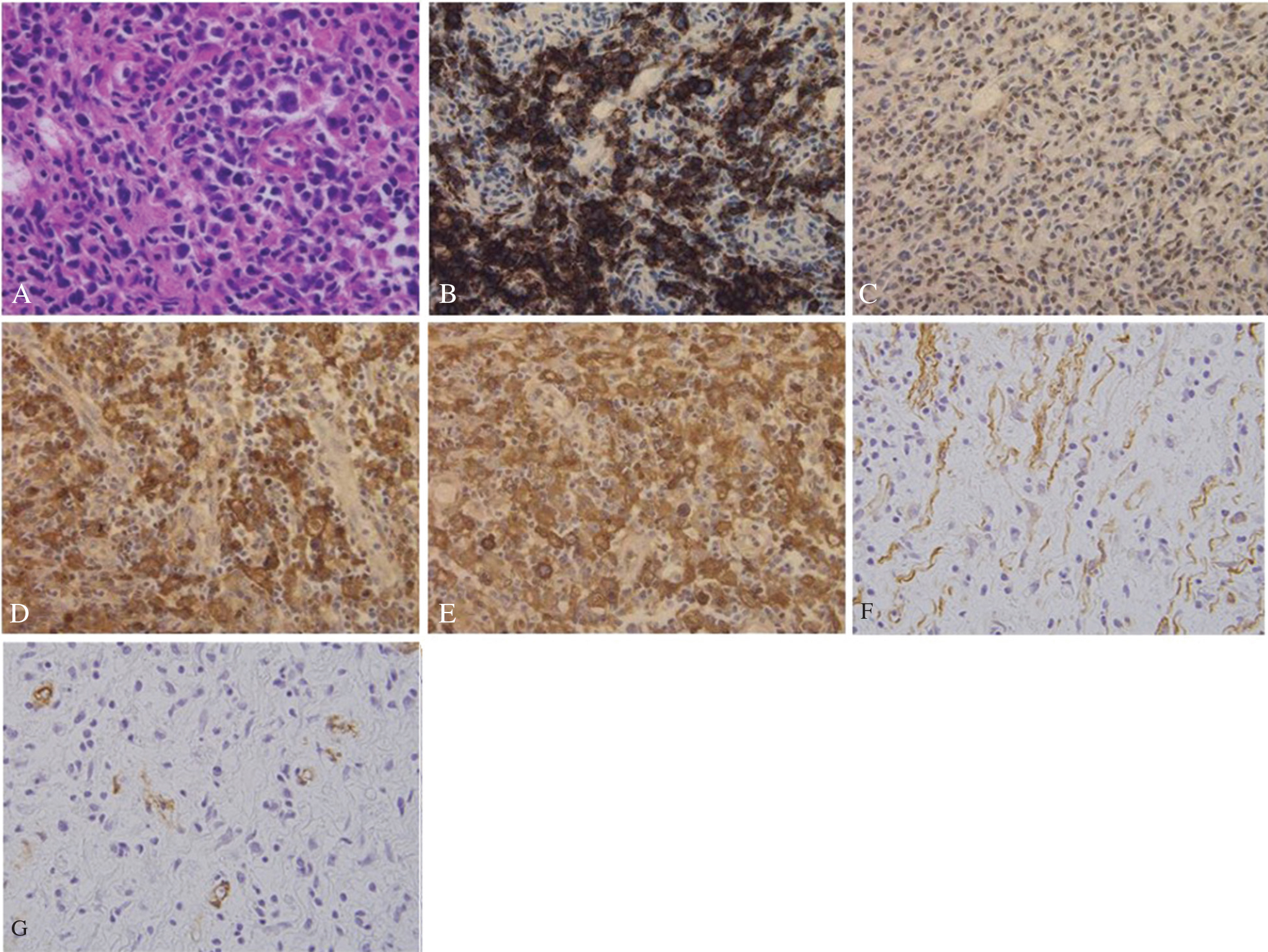
Hematoxylin and eosin staining shows that the paracortical area contains mature plasma cells, plasmacytoid cells, immunoblasts, small and medium-sized lymphocytes, and scattered spindle-shaped cells. Note a Hodgkin-like cell. (A) Magnification, ×100. Immunohistochemical studies demonstrate the mixed nature of the small and medium-sized lymphocytes (B; CD20, C; CD3), whereas the immunoblasts are usually CD20-positive. (B) Magnification, ×100. Immunostaining for the light chain determinant of the immunoglobulins demonstrates the polytypic nature of the plasma cells and their precursors, kappa (E) and (F) lambda. Magnification, ×100. The majority of the spindle cells are positive for vimentin (F), with some positive for muscle-specific actin (G). Magnification, ×100.
There were no Epstein-Barr Virus Encoded Small RNA (EBER)-positive cells in the lesion. A positron emission tomography scan revealed no other systemic malignant tumors, including malignant lymphoma (data not shown).
After the operation, the patient’s symptoms improved gradually without any additional treatments. The motor function of her right leg recovered to almost the normal level within 4 weeks after the operation. MR images taken 1-month after surgery showed spontaneous shrinkage of the tumor (Fig. 4A), and the tumor became undetectable at one year (Fig. 4B). Recurrence of the tumor was not detected on MR images at 3-year follow-up.

MRI shows shrinking of the mass lesion one month after the operation (A), and no mass lesion is found at the corresponding level one year after the operation (B).
The pathogenesis of IPT remains a matter of debate. Some IPTs develop as satellite lesions associated with malignant tumors or tuberculosis. The multiplicity of sites that can be involved suggests no particular route of entry or any specific agents. Prior surgery, trauma, or immune disturbances, in addition to infection, are included as possible etiologies.13) Like most of the previously-reported cases of IPT, none of these factors were found in the present case.
IPT is known to be an inflammatory lesion.14) Like every inflammatory process, the histopathological pattern of IPT changes with time, ranging from densely cellular lesions with neutrophil chemotactic activity in early IPT, to less cellular and more fibroblastic masses in end-stage lesions.14) The present case corresponds to middle-stage, as a significant portion of the tissue was lymphocytes, with a tiny portion of cells positive for smooth muscle actin and/or vimentin. The presence of these smooth muscle actin and/or vimentin-positive myofibroblastic cells implies that this case was in the phase of resolution of the inflammatory response.15)
The location and shape of IPT in the present case were distinct from those of IPTs in previous reports. In cases of CNS involvement, IPT usually arises from intracranial dura mater and rarely from intraparenchymatous locations.16,17) Cases of IPT that originate in the spinal canal are primarily generated in the spinal cord meninges4) and rarely in spinal cord parenchyma.14) In the present case, however, the pseudotumors were formed with swollen nerve roots of the cauda equina adhering to each other. Because of these pathological characteristics, the preoperative radiographic findings and the operative procedures were different from those of previous IPT cases.
Preoperative MR images and CT myelography demonstrated the unique shape of the tumor, resembling a jellyfish, which is rarely seen in other tumors. The findings that the lesion moved with changes in the patient’s position implies that the lesion adhered to these nerve roots. According to the previous reports, there are no distinctive findings on diagnostic imaging of IPTs. MRI with contrast enhancement demonstrates a dense and homogeneous mass, as seen in other tumor lesions such as lymphoma, metastatic tumor, schwannoma, ependymoma, multiple myeloma, or meningioma.18) A definite diagnosis can be made on the basis of postoperative histopathological studies. The finding that this tumor-like lesion resembled a jellyfish implied that the affected area involved several nerve roots. To the best of our knowledge, this has never been seen in other case reports of IPTs. This finding on diagnostic imaging may be a clue to the type of IPT in the present case.
The therapeutic strategy adopted for the IPT caused by the present pathology was different from that of the IPTs reported previously.6,13,19) In the present case, no tumor could be isolated, and tumor resection was abandoned for biopsy, keeping damage to the nerve roots at a minimum. Although no other treatment, including radiotherapy, chemotherapy, or steroid therapy, was given after the operation, the lesion disappeared spontaneously. In locations such as liver or lung, natural regression of IPTs has been reported previously.20,21)Since the mechanisms of these cases of natural regression are not well understood, conservative therapy is not commonly recommended as the first choice for IPT in these regions, and resection is suggested as the initial treatment for IPT patients with mass effect symptoms or where tumor mass is easily excised.20) The same course of treatment should be adopted in the CNS, where natural regression of IPTs has never been previously reported. Although biopsy of the lesion may be one of the factors, the mechanism of the spontaneous clinical improvement and radiographic shrinking in the present case remains to be clarified.
IPT should be considered a possible etiology of intradural extramedullary spinal tumors, especially when it appears to involve several nerve roots on diagnostic imaging. Surgery is required for diagnosis. Since spontaneous regression of an IPT may occur, conservative treatment can be a treatment of choice, depending on the intraoperative findings and the postoperative clinical course. However, since recurrence is common, long-term follow-up should be performed.
The authors received no financial support for this study. They have no personal, financial, or institutional interests in any of the materials or methods described in this article.