2020 Volume 7 Issue 4 Pages 151-155
2020 Volume 7 Issue 4 Pages 151-155
Cerebral hyperperfusion syndrome (CHS) is a potentially devastating complication of carotid endarterectomy (CEA). Early detection and treatment of hyperperfusion are important before the condition develops into CHS. We herein present a case involving a 65-year-old female with severe right internal carotid artery (ICA) stenosis, who experienced hyperperfusion after right CEA. During the postoperative course, changes in the resting cerebral blood flow (rCBF) were evaluated using single-photon emission computed tomography (SPECT), and were found to correlate with the changes in the signal intensity of cortical arteries, cortical veins, and perilateral ventricular veins of the right middle cerebral artery (MCA) territory on susceptibility-weighted imaging (SWI). SWI showed a prominent hyperintensity of cortical arteries in the right MCA territory at postoperative day 1 (POD1), but the hyperintensity gradually decreased over time and became indistinct by POD48. As for cortical veins and perilateral ventricular veins, SWI showed an increased signal intensity of these veins during the peak of rCBF on POD1, but later, the signal intensity decreased as rCBF decreased on POD5. The signal intensity of cortical veins and perilateral ventricular veins finally returned to normal on POD9. Those SWI findings could be related to an impairment of cerebral autoregulation and the resulting hyperperfusion. SWI could be potentially useful as an additional tool in the evaluation of hyperperfusion.
Cerebral hyperperfusion syndrome (CHS) after carotid endarterectomy (CEA) is characterized by unilateral headache, facial and ocular pain, seizures, as well as focal neurological deficits due to cerebral edema or intracerebral hemorrhage.1) Intracerebral hemorrhage associated with CHS has been reported to lead to high mortality and morbidity.1) Although CHS after CEA is rare, cerebral hyperperfusion must be detected as early as possible and some intervention has to be performed before the condition develops into CHS. While positron emission tomography and single-photon emission computed tomography (SPECT) are the gold standards for the evaluation of cerebral hyperperfusion, both methods are costly and require the use of radionuclides; therefore, those are not convenient in cases that need frequent or immediate examination. On the other hand, useful hemodynamic information can also be obtained from susceptibility-weighted imaging (SWI), which is a non-contrast magnetic resonance imaging (MRI) sequence characterized by its sensitivity to paramagnetic substances.2,3) We report a case of cerebral hyperperfusion after CEA, in which the patient presented with dynamic changes in the SWI signal intensities of unilateral cortical arteries, cortical veins, and perilateral ventricular veins, which were correlated with changes in the resting cerebral blood flow (rCBF) as evaluated by SPECT. Although there were some reports of SWI findings related to luxury perfusion after cerebral infarction and focal cerebral hyperperfusion during the ictal state of an epileptic seizure,4–6) this is the first report of SWI findings related to cerebral hyperperfusion after CEA.
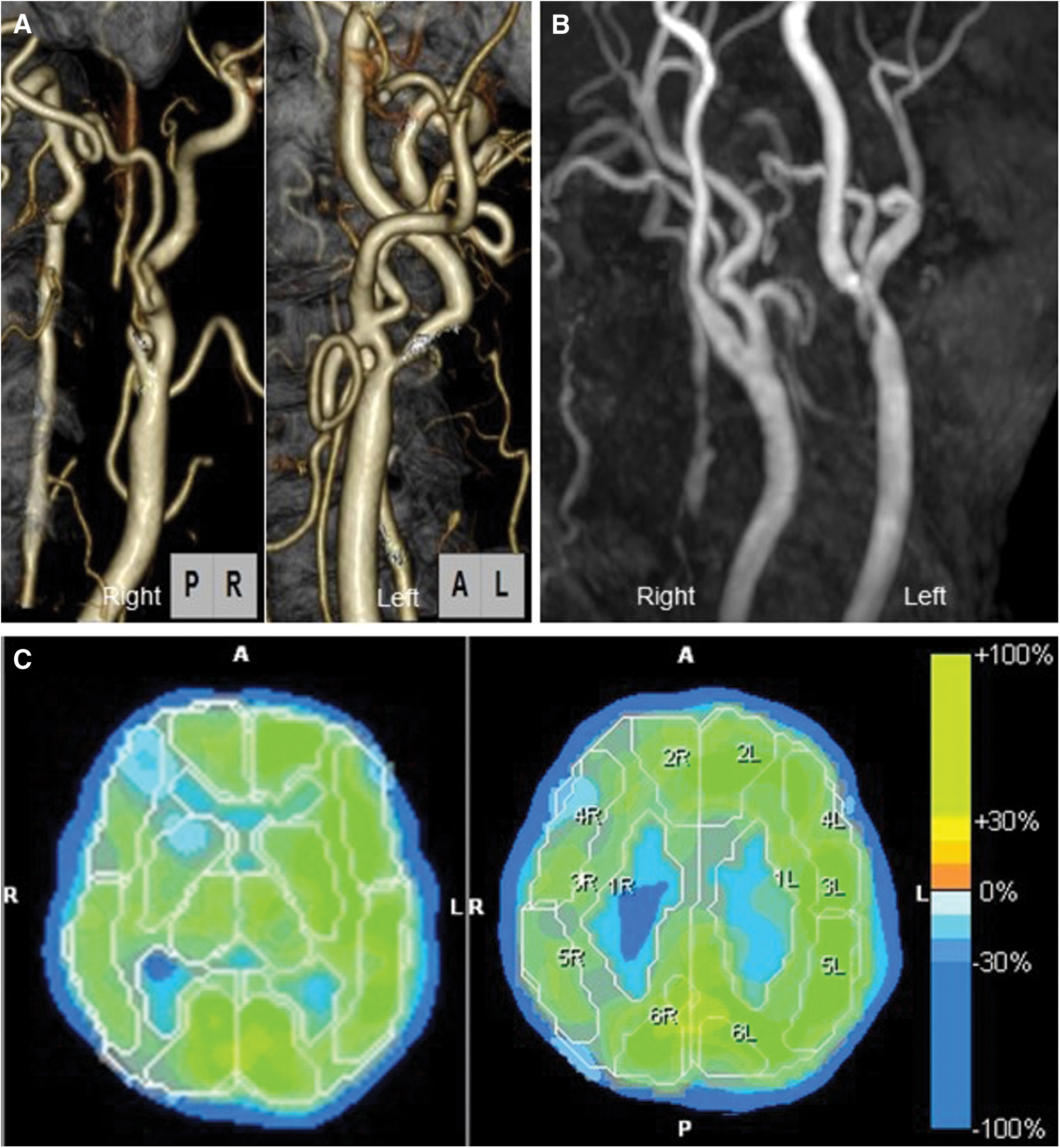
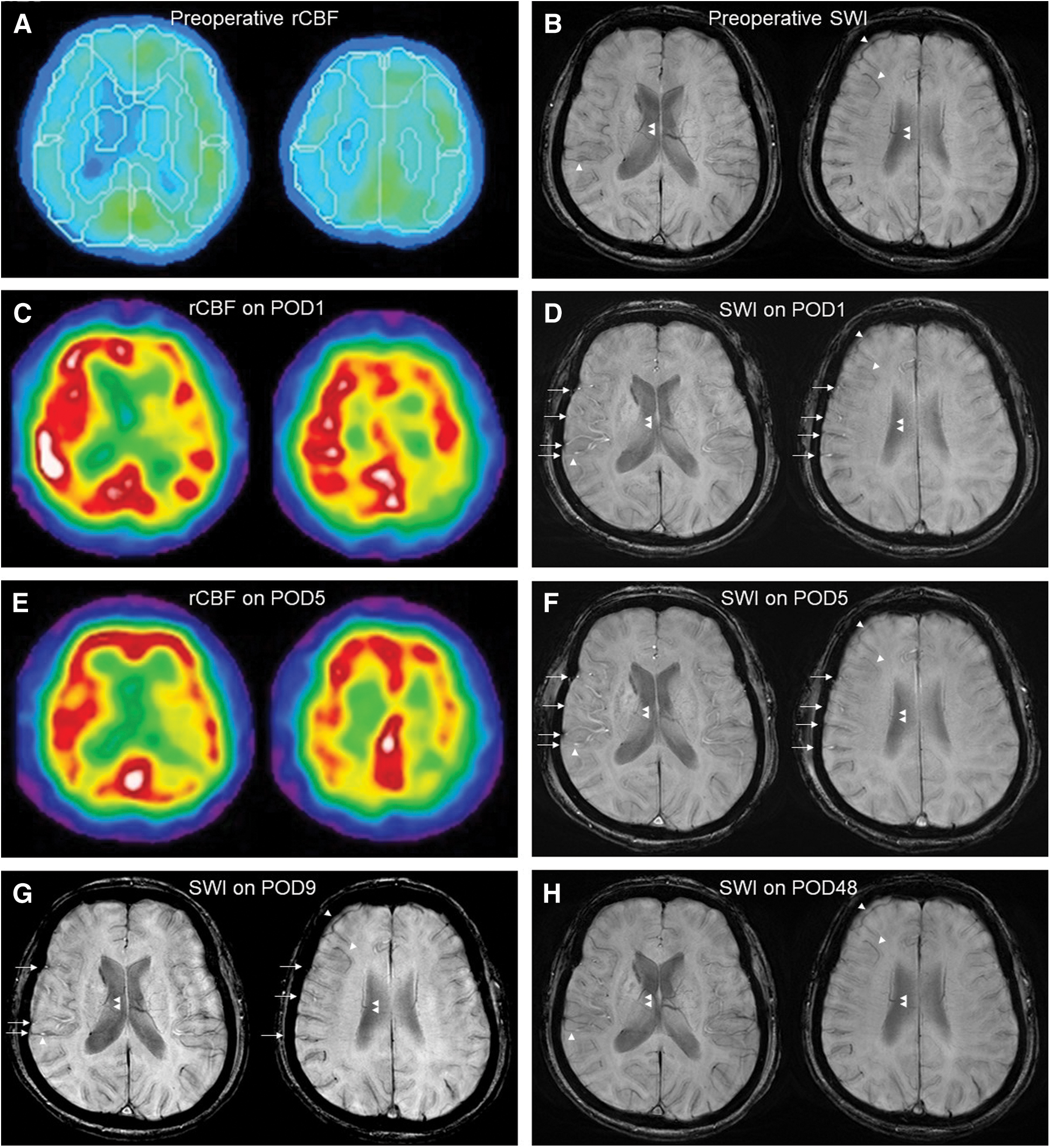
A 65-year-old woman with hypertension underwent right CEA due to severe asymptomatic stenosis of the right internal carotid artery (ICA). Preoperative cervical computed tomography angiography showed severe right ICA stenosis (Fig. 1A). The rate of stenosis was 99% in the right ICA and 50% in the left ICA, as measured using the North American Symptomatic Carotid Endarterectomy Trial Collaborators method. Preoperative SPECT with N-isopropyl [123I]-p-iodoamphetamine (IMP-SPECT) with and without acetazolamide showed an impaired cerebral vascular reserve capacity and a markedly decreased rCBF (Figs. 1C and 2A). MRI (3-T Siemens Skyra; Siemens, Erlangen, Germany) showed no acute infarction and magnetic resonance angiography (MRA) showed no residual ICA stenosis at postoperative day 1 (POD1) (Fig. 1B). Source images were used to analyze SWI. SWI showed a markedly increased intensity of the cortical arteries, cortical veins, and perilateral ventricular veins in the right middle cerebral artery (MCA) territory compared with the left side and preoperative SWI findings (Figs. 2B and 2D). IMP-SPECT on POD1 showed significantly increased rCBF in the right MCA territory compared with preoperative rCBF, and the rCBF on POD1 in some areas of the right MCA territory was twofold higher than the preoperative rCBF (Figs. 2A and 2C). The ratio of rCBF (right/left) was increased on POD1 compared with preoperative values (1.34 vs. 0.86 in the cortex of the MCA territory, 1.20 vs. 0.89 in the basal ganglia; POD1 vs. preoperative). To prevent the patient's condition from developing into HPS, she was placed in a propofol-induced coma with intensive arterial systolic blood pressure control. IMP-SPECT on POD5 showed that the right/left ratio of the rCBF had decreased in compared to POD1 (1.14 in the MCA territory and 0.97 in the basal ganglia on POD5) (Fig. 2E). SWI on POD5 showed that the laterality of the prominent hyperintensity of the cortical artery still remained present, whereas the laterality of signal intensities of cortical veins and perilateral ventricular veins had decreased (Fig. 2F). Then, the sedation was stopped on POD7. Although the laterality of the signal intensities of cortical veins and perilateral ventricular veins of the right MCA territory had disappeared, the laterality of the prominent hyperintensity of the cortical artery on SWI decreased on POD9 (Fig. 2G), and subsequently, the patient was discharged from our hospital without neurological deficits on POD23. SWI findings on POD48 showed no laterality of the signal intensities of cortical arteries or those of the cortical veins and the perilateral ventricular veins of the right MCA territory (Fig. 2H).
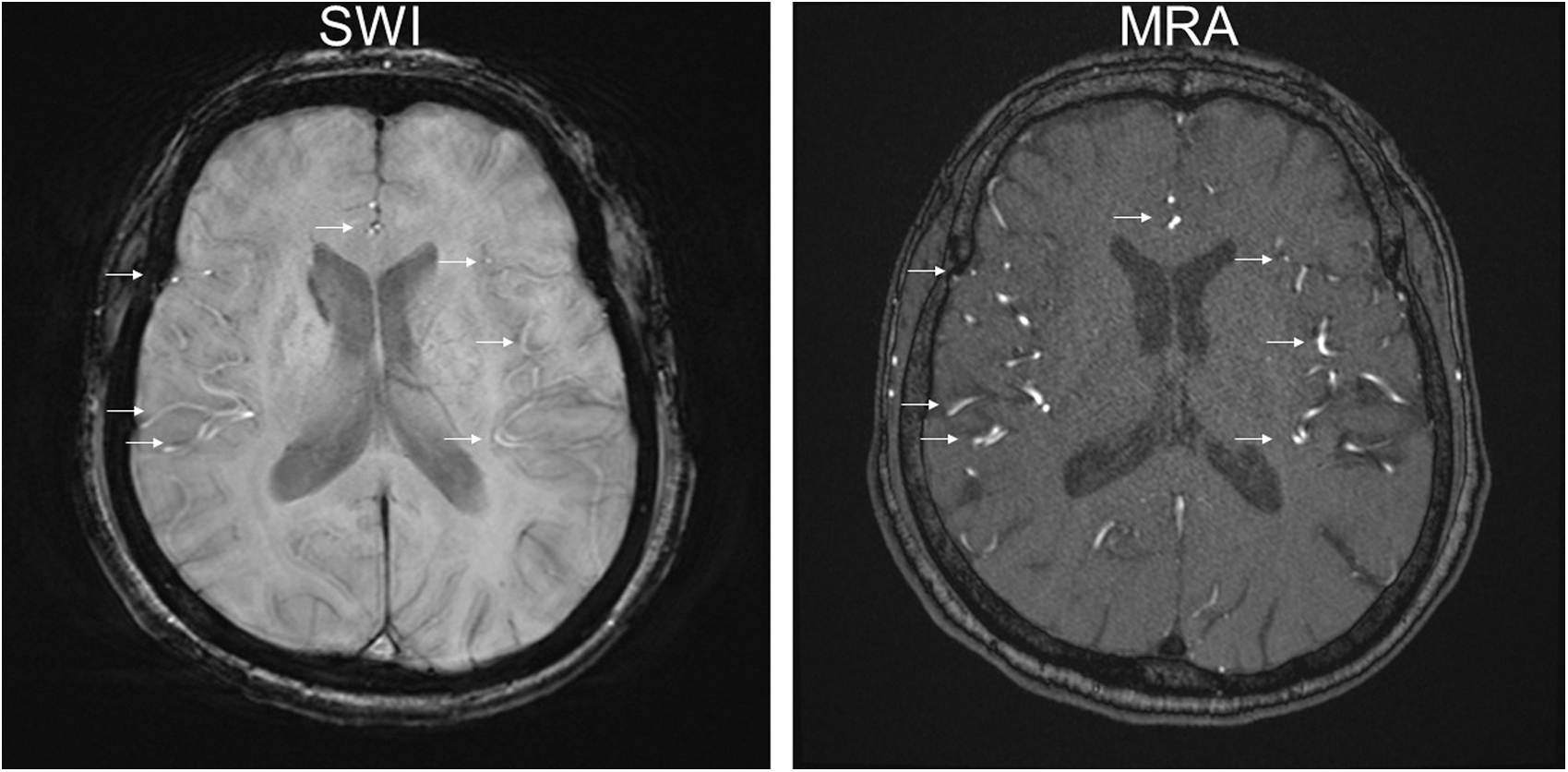
In this case, the changes in rCBF correlated with the changes in the SWI signal intensities of the cortical arteries, cortical veins, and perilateral ventricular veins in the right MCA territory before and after CEA. The magnetic susceptibility of blood is linearly scaled with blood oxygen saturation on SWI.7) In SWI, normal veins show hypointensity due to high levels of deoxyhemoglobin in venous blood, and arteries have higher signal intensity than normal veins due to low levels of deoxyhemoglobin in the arterial blood and time-of-flight inflow enhancement (Fig. 3).8) Previous reports have shown that on POD1 after carotid artery stenting and CEA, a bilateral increase in CBF, shortening of mean vascular transit time in ipsilateral MCA territory and an ipsilateral decrease in oxygen extraction fraction occurred due to restoration of normal perfusion pressure.9,10) In case of hyperperfusion, abnormally increased CBF and decreased oxygen extraction fraction may not allow for sufficient elevation of deoxyhemoglobin levels. This is consistent with previous reports of the presence of red veins in patients with superficial temporal artery (STA)-MCA anastomosis who subsequently experienced intracerebral hemorrhage due to hyperperfusion11) and focal hyperperfusion during the ictal state of an epileptic seizure.4,5) In our case, the elevated signal intensity of perilateral ventricular veins may have been due to decreased levels of deoxyhemoglobin caused by hyperperfusion.
A prominent SWI hyperintensity of the cortical arteries of the right MCA territory was found on POD1 and POD5, showing a laterality which later decreased on POD9 and eventually disappeared on POD48. In SWI, arteries with high-velocity flow are depicted as hyperintensity because of time-of-flight inflow enhancement.8) The blood flow velocity of MCA was increased in hyperperfusion.12) This change of arterial flow velocity could affect the time-of-flight inflow enhancement13); therefore, the intensities of cortical arteries of right MCA territory on SWI increased in hyperperfusion. Patients with chronic ischemia have often been reported to show impaired cerebrovascular autoregulation.14) Like the case described in the present study, chronic ischemia with severely impaired CVR may cause vasoparalysis. As a result, cerebral artery branches in the right MCA territory could be unable to respond quickly enough to the rapid restoration of normal perfusion pressure after CEA. CBF was also reported to increase rapidly and peak in the acute phase, whereas cerebral vascular reserve gradually improved over time after carotid artery stenting.15) This is consistent with our findings, and the changes in the signal intensities of cortical artery might indicate the recovery of cerebrovascular autoregulation.
Changes in the SWI signal intensity of cortical arteries, cortical veins, and perilateral ventricular veins in an ipsilateral MCA territory after CEA may provide valuable information regarding hyperperfusion after CEA. Further investigations using larger sample sizes will be required to assess the relationship between hyperperfusion and the changes in the signal intensities of cortical arteries, cortical veins, and perilateral ventricular veins in the MCA territory after CEA.
The authors extend their gratitude to all personnel at Nagasaki Prefecture Shimabara Hospital who contributed to this study, as well as to the patient who kindly gave permission for her data to be utilized in this publication, and to Dr. Herizo Fabien Rafidinarivo and Jack Middleton who edited the manuscript. The authors would also like to thank Minoru Morikawa, Department of Radiological Sciences, Graduate School of Biomedical Sciences, Nagasaki University, for helpful comments regarding the mechanisms of MRI.
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Informed consent has been obtained from the patient for publication of this manuscript.
The authors declare no conflict of interest.