2021 Volume 8 Issue 1 Pages 1-5
2021 Volume 8 Issue 1 Pages 1-5
Exertional vertebrobasilar insufficiency (VBI) secondary to the non-atherosclerotic cause is uncommon. We herein report the case of a patient who developed exertional VBI long after extracranial right vertebral artery (VA) dissection. At the time of dissection, the right VA was completely occluded near its origin, but the distal flow was compensated by the collateral flow from the right deep cervical artery (DCA). After conservative management, the patient was discharged without neurologic deficit. Six years later, he developed recurrent VBI in association with the exertion of his right shoulder. A vascular evaluation revealed that the right proximal VA was still occluded, and there was no evidence of right subclavian artery lesions. The intracranial right VA flow was markedly reduced during the period, while branches of the right DCA were given off to the muscles of the right shoulder and neck. Then, occipital artery (OA)-posterior inferior cerebellar artery (PICA) anastomosis was performed. Intraoperative indocyanine green videoangiography (ICG) confirmed that the flow of the right PICA was predominantly supplied from the compensatory flow from the contralateral VA, and the antegrade flow in the right VA was clearly delayed in comparison to that of the left VA while there were prominent branches providing the blood flow to the medulla oblongata. After the anastomosis, these medullary branches provided the blood flow to the medulla oblongata more quickly and extensively than before. Postoperatively, VBI no longer occurred even after exertion. Surgical revascularization can be a viable option in the treatment of refractory VBI of the non-atherosclerotic cause.
Vertebrobasilar insufficiency (VBI) is an ischemia of the posterior circulation that typically occurs in patients with atherosclerotic occlusive disease. Because the infarction in the territory of the posterior circulation is likely to be catastrophic, resolution of hemodynamic compromise is required for symptomatic cases.1) We herein report the case of a patient who developed refractory VBI symptoms associated with exertion of the right shoulder 6 years after the extracranial vertebral artery (VA) dissection, which was successfully treated with occipital artery (OA)-posterior inferior cerebellar artery (PICA) anastomosis.
The patient was a 47-year-old right-handed man initially presented acute right lower neck pain followed by vertigo in 2013. One week after the ictus, magnetic resonance imaging (MRI) revealed acute infarction in the territory of the right PICA while MR angiography confirmed the existence of flow signal in the VA and PICA of the right side (Fig. 1). Catheter angiography revealed complete occlusion of the proximal right VA, and the distal right VA was supplied by collateral flow from the muscle branch at the craniocervical junction. After conservative management, he was discharged without any neurological deficit.
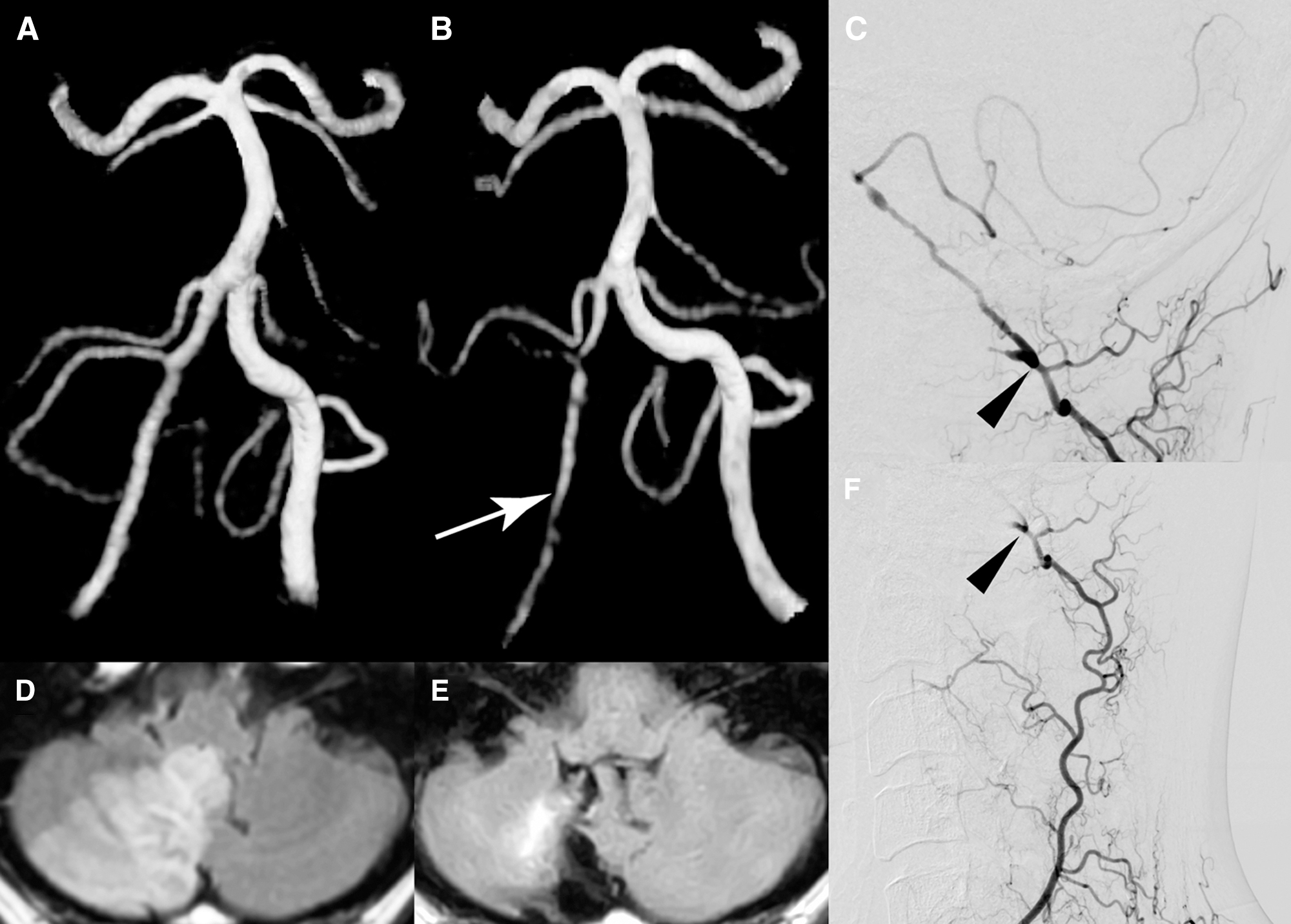
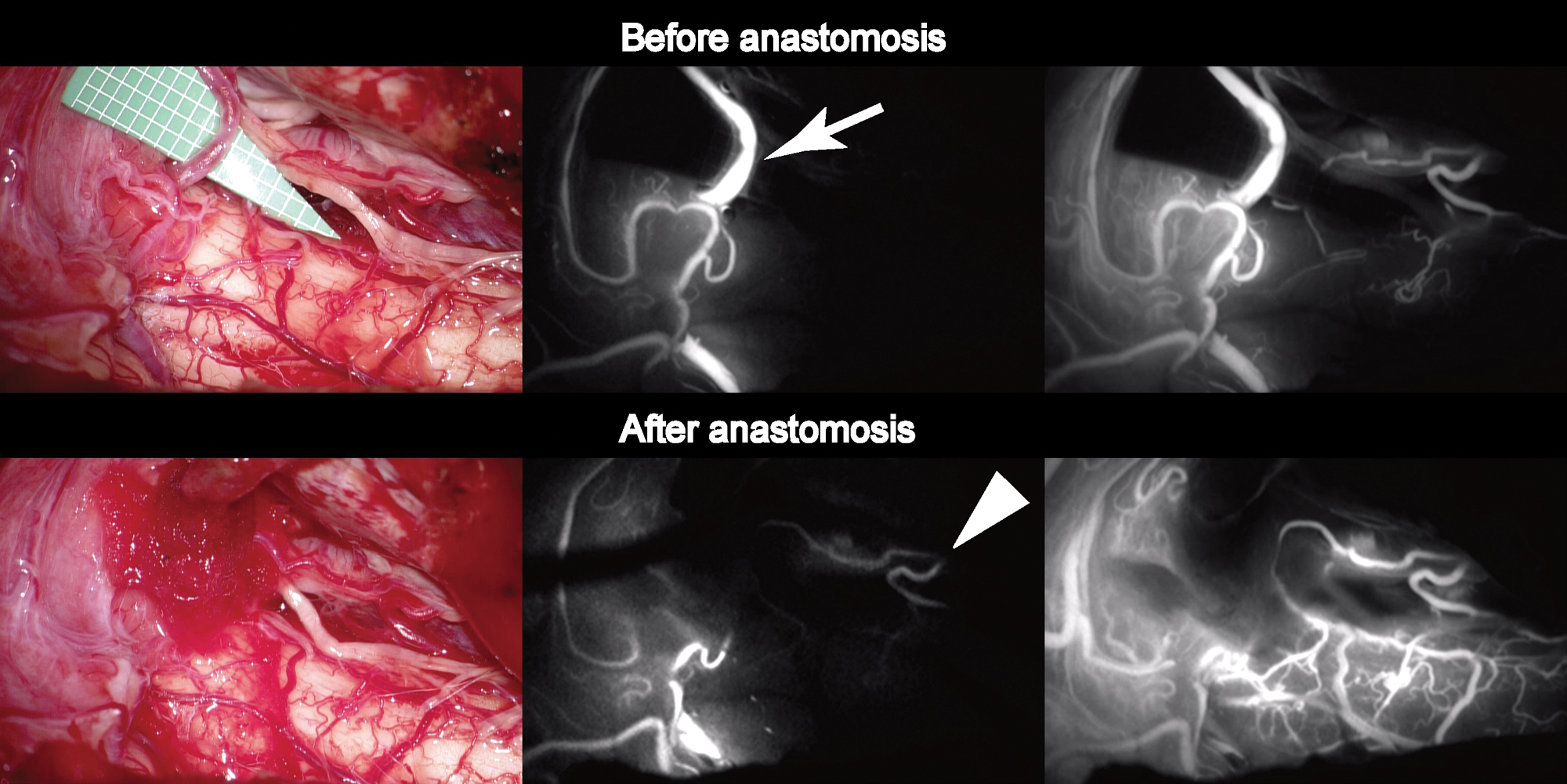
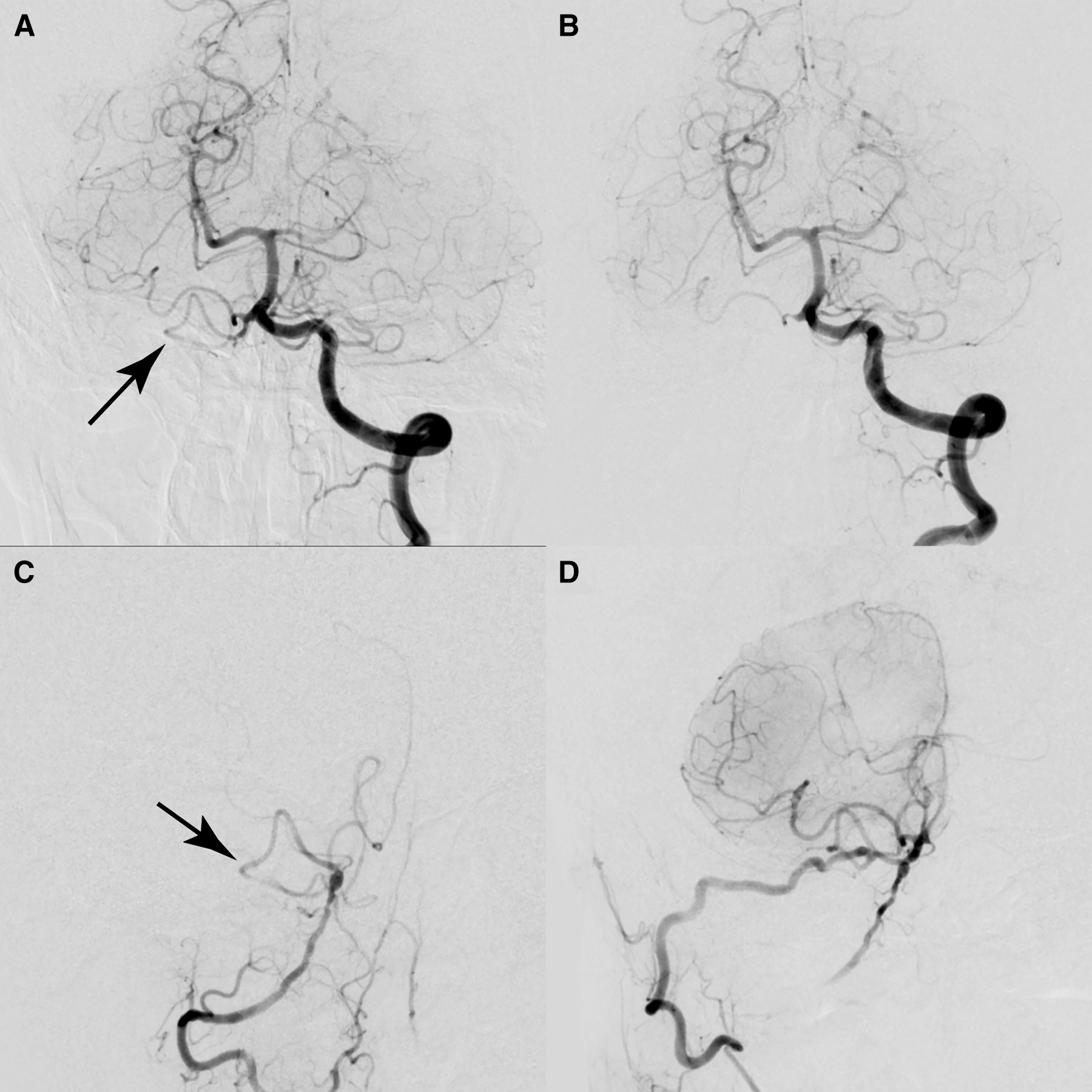
Six years later, while working at a laundry, which required vigorous use of the right shoulder for ironing, he started to develop repeated drop attack in association with exertion. Once these attacks occurred, they would gradually improve after he ceased exertion and lay down. The patient denied that these attacks were induced by specific head rotation. Because the episodes were reproducible and had increased in frequency, he went to a private clinic and was referred to our hospital. On admission, neurological and physical (including blood pressure and heart rate) examinations revealed no abnormalities. MR angiography revealed that the intracranial right VA was narrowing and the right PICA flow was almost disappearing (Fig. 1). Axial T2-weighted images showed no abnormal morphological changes of outer contour in the intracranial right VA. Catheter angiography demonstrated that the right VA remained completely occluded near the origin, and there was no evidence of right subclavian artery stenosis or occlusion. Selective injection from the right costo-cervical trunk revealed well-developed deep cervical artery (DCA) giving off numerous branches around the muscles of the right shoulder and neck, with prominent connection to the remnant right VA at the craniovertebral junction (Fig. 1). Based on these findings, the patient’s reproducible VBI was considered to be due to both the progressive narrowing of the intracranial right VA and exertion-related reduction of collateral flow from the DCA. We proposed several therapeutic options to the patient, including conservative management and surgical revascularization. After a careful discussion, the patient chose to undergo surgical revascularization. Right suboccipital craniotomy was performed under general anesthesia. To facilitate an upward surgical trajectory, a C-1 laminectomy was added. Due to the previous infarction, the cerebellar tonsil was slightly atrophied. Intraoperative indocyanine green videoangiography (ICG) was used before anastomosis to evaluate the hemodynamics (Fig. 2). The flow of the right PICA was predominantly provided from the compensatory flow from the contralateral VA, and the antegrade flow in the right VA was clearly delayed in comparison to that of the left VA while there were prominent branches providing the blood flow to the medulla oblongata. The harvested OA was anastomosed to a caudal loop of the right PICA using interrupted 10-0 nylon sutures. After anastomosis, the prominent medullary branches from the right VA provided the blood flow to the medulla oblongata more quickly and extensively than before. No periprocedural complications were noted and the patient’s postoperative course was uneventful. Postoperative angiography showed good patency of the bypass (Fig. 3). The patient was discharged with a modified Rankin Scale of 0, and the exertional presyncope resolved during the follow-up period.
Although VBI can be triggered by various conditions, reproducible attacks secondary to specific exertion (right shoulder exertion in this case) are uncommon. Furthermore, the development of VBI 6 years after cervical VA occlusion, as was observed in this case, was atypical.
Over the long term, the antegrade flow in the proximal intracranial right VA was obviously decreased in this case. In general, the infarcted brain area no longer requires the same blood supply as before; thus, the flow demand of the right PICA and proximal intracranial right VA in this case was presumably reduced after cervical right VA occlusion and right cerebellar infarction. Slow progression of the vessel narrowing, or vascular remodeling, is sometimes observed as a result of chronic low perfusion pressure in a downstream vessel. It is therefore plausible that the right VA flow was a reason for the decrease, but is sufficient to stabilize posterior circulation at rest.
However, shoulder exertion promotes blood demand in the muscle branches of the DCA, possibly decreasing the collateral supply, which triggered VBI in this case. Because the right anterior inferior cerebellar artery of the present patient did not originate from the basilar artery but branched between the VA union and right PICA, it potentially stole part of the compensatory back flow from the VA union. Due to this anatomical variation, the patient might have been vulnerable to the decreased antegrade collateral flow in the right VA that supplied prominent medullary branches.
Although the drop attack observed in this case was resolved after the successful revascularization to the right PICA, it was unlikely to be explained by the blood flow increase in the territory of right PICA alone. We speculated that the main cause of the preoperative symptom was attributable to the hypoperfusion of medulla oblongata rather than that of right cerebellar hemisphere supplied by the right PICA. As shown in Fig. 2, hemodynamic improvement was more clearly occurred in medulla oblongata than right cerebellar hemisphere, and this finding may account for our hypothesis.
The VBI of the present patient was unlikely to be explained by a thromboembolic mechanism; thus, there was no justification anti-thrombotic therapy in this case. Feasibility and safety of endovascular reopening of the chronically occluded right VA is unknown. Surgical revascularization seemed to be viable option to safely resolve the underlying pathophysiology in this case. Selection of appropriate candidates for aggressive intervention is desired and quantitative flow measurement may be promising for predict treatment outcome1,2); however, the imaging tool may not be feasible in the daily clinical practice.
Surgical revascularization for stenosis of vertebrobasilar system prevents ischemic attacks.2–6) In a single center study, 30 patients with vertebrobasilar occlusive disease who underwent superficial temporal artery-superior cerebellar artery anastomosis showed excellent outcome at discharge, with morbidity and mortality of 0% and 3.3%, respectively.6) In a series of nine patients who had undergone OA-PICA anastomosis, patency rate was as high as 100%, whereas one patient had cerebellar infarction despite patent graft.7) Potential complications of the OA-PICA anastomosis include cerebellar infarction secondary to graft occlusion, wound healing, and cerebrospinal fluid leakage.8,9)
The technical complexity of the OA-PICA anastomosis depends on the clinical anastomotic depth, and is influenced by the presence or absence of the caudal loop of the PICA. In one cadaveric study that measured the distance between the site of anastomosis and the point of entry for surgical instruments, the depth of anastomosis in the caudal loop segment (7.6 ± 2.4 mm) was found to be significantly shallower than that in the lateral medullary segment (16.5 ± 3.0 mm) and telovelotonsillar segment (17.1 ± 2.9 mm), respectively.4) While the caudal loop is thought to be the optimal recipient site for OA-PICA anastomosis, it sometimes runs horizontally without forming it. The individual course of the PICA should be precisely evaluated before surgery, and 3DCTA can be a powerful tool for this purpose.
The authors have no conflicts of interest to declare.