2021 年 8 巻 1 号 p. 85-88
2021 年 8 巻 1 号 p. 85-88
Ischemic stroke associated with intracranial aneurysm is rare but potentially happens because of emboli originating from aneurysm sac or aneurysmal thrombosis extension to the parent artery. We describe two patients who present subarachnoid hemorrhage (SAH) soon after ischemic stroke. Case 1. A 51-year-old woman with a history of multiple endovascular therapy for ruptured basilar top aneurysm presented with double vision. Magnetic resonance imaging (MRI) revealed infarcts in the right thalamus and left occipital cortex. Four days after ischemic stroke, she suffered from sudden onset headache, computed tomography (CT) showed diffuse SAH with intraventricular hemorrhage. Case 2. A 62-year-old man presented with right facial palsy and sensory disorder. MRI revealed an infarct in the left pons. Four days after ischemic stroke, he became comatose and CT showed diffuse SAH. Both cases develop ischemic stroke adjacent to the aneurysms and subsequently cause devasting aneurysm rupture, suggesting ischemic stroke as a warning sign of aneurysm rupture. In such cases, early treatment of the aneurysm should be considered.
Ischemic stroke associated with intracranial aneurysms is rare but potentially happens because of emboli originating from aneurysm sac or aneurysmal thrombosis extension to the parent artery.1) However, ischemic stroke adjacent to the aneurysms is not commonly considered as the risk of rupture of aneurysms. We herein describe two cases of presenting subarachnoid hemorrhage (SAH) following minor ischemic stroke which may suggest ischemic stroke as a warning sign of aneurysm rupture.
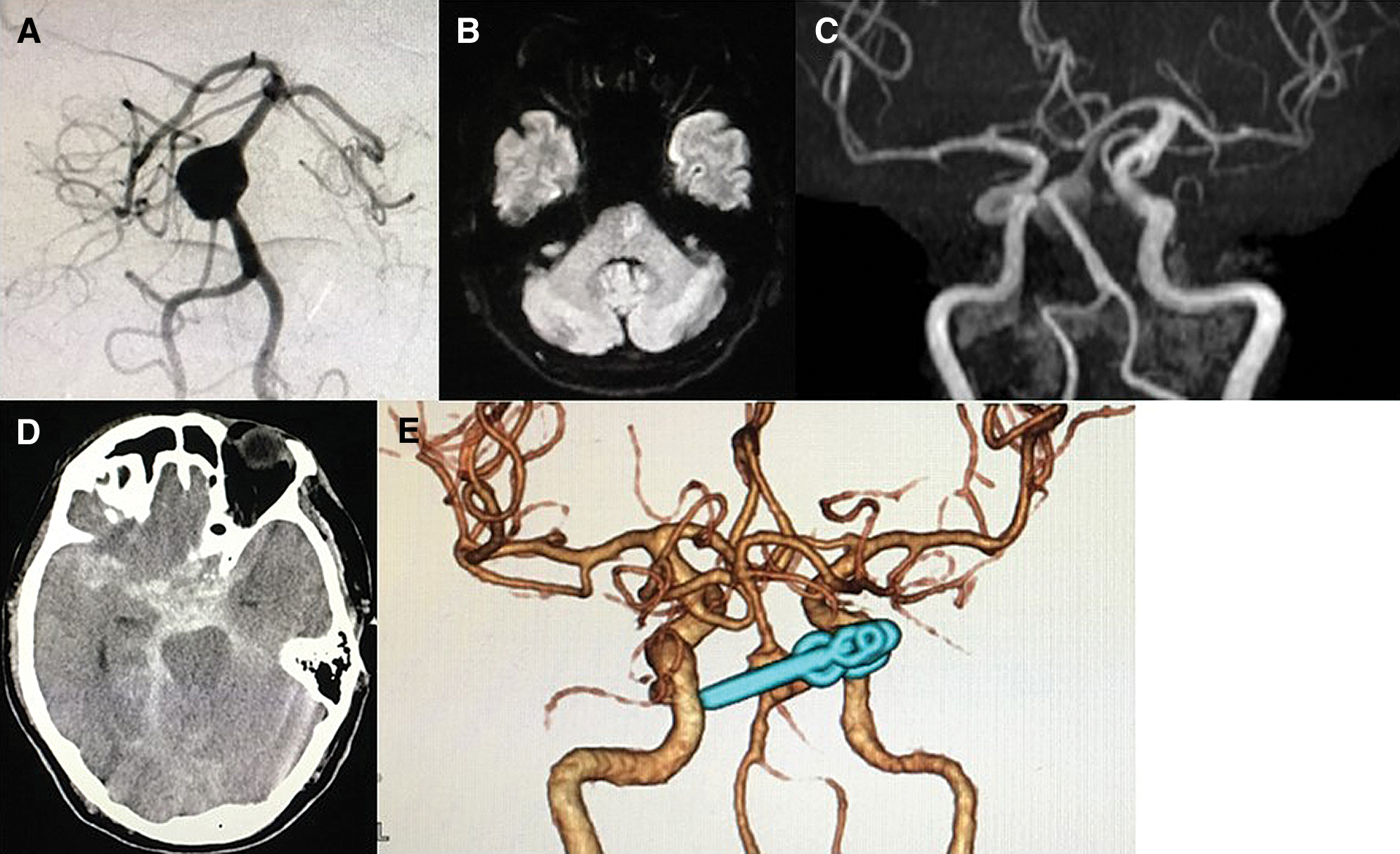
A 51-year-old woman with a history of hypertension developed SAH due to ruptured basilar top aneurysm 6 years ago. Stent-assisted coil embolization was performed multiple times at another hospital and ended with neck remnant (Figs. 1A–1C), but she recovered without any deficit. She presented with double vision lasting for a week. Neurological examination showed adduction disorder of the right eye, diplopia, and mild dysarthria. Magnetic resonance imaging (MRI) revealed infarcts in the right thalamus and left occipital cortex (Fig. 1D). Dual antiplatelet therapy (DAPT) and argatroban were initiated. On the fourth day of hospitalization, she became unresponsive and bilateral pupil dilation after complaining of a sudden and severe headache (The Glasgow Coma Scale score was 3) and her Hunt and Hess grade was 5. A head computed tomography (CT) showed diffuse SAH with intraventricular hemorrhage (Fig. 1E). The patient underwent emergent endoscopic hematoma evacuation and ventricular drainage followed by coil embolization. The apparent enlargement of body filling of residual aneurysm was observed. Unfortunately, the death occurred 6 days after surgery (Figs. 1F and 1G).

A 62-year-old man with a history of atrial fibrillation and hyperthyroidism was referred to our hospital for a diagnosis of a large fusiform aneurysm of the basilar artery incidentally detected by MRI and confirmed by cerebral angiography (Fig. 2A). Two months later, he presented with right facial palsy and sensory disorder. Neurological examination showed mild dysarthria and dysesthesia of the right leg. MRI revealed an infarct in the left pons (Fig. 2B and 2C). DAPT and argatroban were initiated, but his hemiplegia was progressed. On the fourth day of hospitalization, he became coma (The Glasgow Coma Scale score was 3) and his Hunt and Hess grade was 5. A head CT showed diffuse SAH (Fig. 2D). We performed ventricular drainage and clipping by the anterior transpetrosal approach (Fig. 2E). Postoperative course was uneventful and he recovered gradually, he was transferred to a rehabilitation hospital by the modified Rankin Scale (mRS) of 4.
We present two cases of SAH following minor ischemic stroke adjacent to unruptured aneurysm. As previously studied, the prevalence of ischemic stroke or transient ischemic attack is 3–6.3% among patients with unruptured intracranial aneurysms (UIA).2,3) There are few reported cases of ruptured aneurysms after cerebral infarction, but it may be encountered in clinical practice. The cause of cerebral infarction caused by UIA due to local extension of the luminal thrombus, distal embolization, and increased mass effect have been implicated as possible mechanism.3)
In our two cases, any of the above possibilities were considered. The cause of stroke for Case 1 is considered the thrombus formation inside the aneurysm or the mass effect of enlarged aneurysm on the perforator. The cause of stroke for Case 2 is considered an enlargement of the aneurysm or progression of dissecting aneurysm. Numerous studies have identified the close relationship between hemodynamic-induced inflammation and rupture of aneurysm. The dysfunction of endothelial cells, smooth muscle cells, macrophages, as well as their secreted cytokines, collectively contribute to the rupture of aneurysm.4,5) Luminal thrombosis, distal embolization, and increased mass effect of aneurysms occur in the process of inflammation of aneurysms. Based on these findings, ischemic stroke in the perforator area adjacent to aneurysms suggests a possibility of a warning sign of the aneurysm rupture because the cause of the aneurysm rupture and ischemic stroke seems similar in terms of inflammatory process. In general, blood pressure keeps high and antithrombotic drugs are administered after cerebral infarction, but these two cases have developed SAH after the start of antithrombotic drugs. Considering the risk of developing SAH, we should be careful to start antithrombotic drugs. In both the cases, SAH develop several days after minor ischemic stroke, suggesting a sign of impending rupture. It is necessary to investigate which cases are at high risk of developing SAH and which cases can be treated with antithrombotic drugs for ischemic infarction around the aneurysm. Both of our cases had poor outcome regardless of intensive treatment, physicians should be alerted to the need for closed follow-up, and early surgical procedure for ischemic stroke adjacent to aneurysms.
SAH after ischemic stroke is a rare but causes serious complications. Ischemic stroke adjacent to unruptured aneurysms suggests a possibility of a warning sign of the aneurysm rupture. Acute management should be undertaken with care regarding antithrombotic drugs and early surgical procedure should be considered.
No authors report any conflicts of interest.