2021 Volume 8 Issue 1 Pages 101-105
2021 Volume 8 Issue 1 Pages 101-105
Langerhans cell histiocytosis (LCH) is a disease characterized by the proliferation of Langerhans cells. Most cases of LCH occur in children, although it can be seen in adults as well. We encountered an adult case of LCH. A 44-year-old woman who was diagnosed as diabetes insipidus underwent a magnetic resonance imaging (MRI) of the head which revealed sellar and suprasellar gadolinium-enhanced mass. Prolactin level was high and cabergoline was prescribed. The size of this mass had reduced, so we supposed the tumor was prolactinoma. However, after 4 years of observation, it had increased once again. The biopsy of pituitary stalk lesion was performed via transcranial approach. The histological diagnosis was initially gangliocytoma. The patient complained of back pain after surgery. Three months after the biopsy, a computed tomography (CT) scan revealed multiple osteolytic lesions throughout the entire body. One of the osteolytic lesions of the skull was removed to determine the diagnosis. The pathological examination of the skull led to a diagnosis of LCH. We concluded retrospectively that the lesion of the pituitary stalk was LCH mimicking gangliocytoma though classical pathological findings were not obtained. In conclusion, LCH should be considered as a differential diagnosis in adult cases of diabetes insipidus with hypothalamic–pituitary lesion.
Langerhans cell histiocytosis (LCH) is characterized by the proliferation and organ infiltration of cells that have morphological and immunohistochemical features of the Langerhans cells.1) LCH is often diagnosed in children although some adult cases of LCH have been reported.2) Adult cases of LCH are prone to be misdiagnosed.3–6) Moreover, pathological diagnosis of LCH is difficult because of their variety.7,8) In this report, we present an adult case of LCH that was diagnosed with a biopsy of the osteolytic lesion of the skull that emerged following a biopsy of the pituitary lesion.
A 44-year-old woman presented polydipsia and polyuria. She was diagnosed as diabetes insipidus at the other clinic, and magnetic resonance imaging (MRI) of her head with gadolinium contrast medium revealed sellar and suprasellar mass with homogenous enhancement (Fig. 1a). Her prolactin levels were also high (56.9 ng/mL), whereas other anterior pituitary hormone levels were normal. She was treated with cabergoline and desmopressin acetate hydrate. A follow-up MRI taken at our hospital showed that the size of her tumor had decreased in size (Fig. 1b). We supposed the tumor was prolactinoma. After 4 years of treatment with cabergoline, the size of the tumor had once again increased (Fig. 1c). The patient complained of visual disturbance, and the ophthalmological examination revealed the right homonymous lower quadrantanopia. Thus, we made the decision to remove the tumor. The differential diagnoses were pituitary adenoma, lymphocytic hypophysitis due to various cause, and LCH. Although an MRI performed 2 days before the surgery showed tumor regression (Fig. 1d), we performed the tumor removal as planned to diagnose the lesion. We found the tumor between the right optic nerve and the internal carotid artery via right front-temporal approach. The tumor was a yellowish, hard mass and the small specimen was removed safely. Her postoperative course was uneventful. The pituitary stalk specimen showed an increase in nerve fibers and several ganglion cell-like cells with proliferation of collagen fiber (Fig. 2). Macrophages which contained hemosiderin deposits were also found. There were no pituitary adenomas, nor normal anterior pituitary tissue. Immunohistochemistry shows that nerve fibers were positive for S100 protein. The suspected histopathological diagnosis was gangliocytoma. Adjuvant therapy was not added.


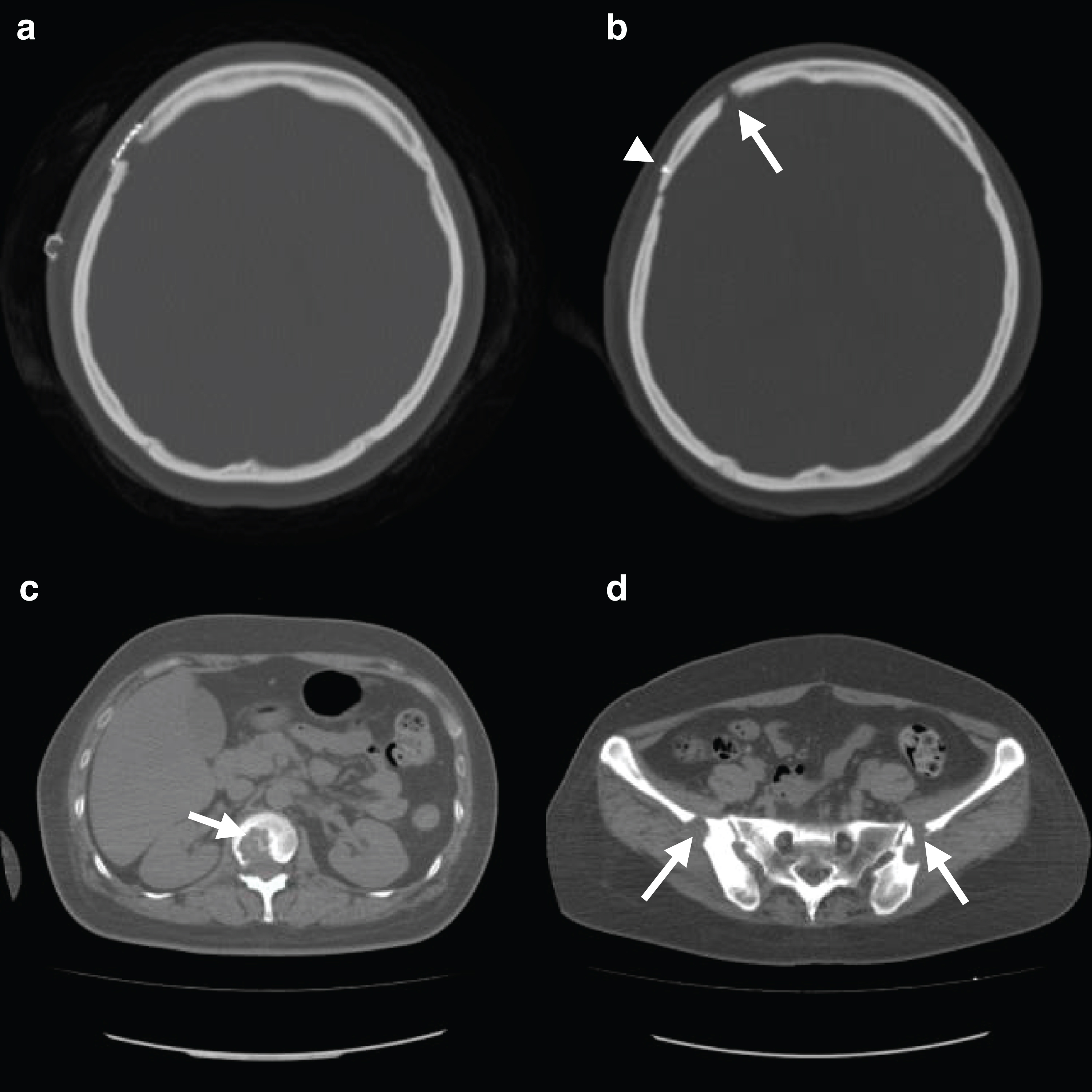
At 1-month post-surgery, the patient complained of back pain. The pain was initially considered to be functional back pain and she was thus put on observation. Her back pain was sustained for 3 months and spread to other joints. A whole-body computed tomography (CT) scan revealed the presence of multiple osteolytic lesions in the skull (Fig. 3b), scapula, vertebra (Fig. 3c), and iliac bone (Fig. 3d). The skull osteolytic lesions were absent just after her biopsy (Fig. 3a). Possible diagnoses of metastasis of malignancy, multiple myeloma, tuberculosis, and LCH were considered. To definitively determine the diagnosis, the osteolytic lesion of skull was biopsied. The skull lesion specimen showed an increase in small cells like lymphocytes (Fig. 4a). There were Langerhans cells with large nuclei, as characterized by their linear groove, as well as eosinophils (Fig. 4b). Immunohistochemistry shows that Langerhans cells expressed CD1a and S100 protein (Figs. 4c and 4d). The histopathological diagnosis of skull lesion was LCH. The patient was prescribed prednisolone and methotrexate for more than 2 years. Her pain subsided, and only prednisolone was continued.

LCH is characterized by the proliferation of Langerhans cells, as determined by morphological and immunohistochemical analysis.1) Most cases of LCH are seen in children although there are some adult cases,2) though adult patients are often misdiagnosed.3–6) First time, we had rarely considered LCH because this patient was 44 years old. Among the cases of hypothalamic–pituitary LCH, central diabetes insipidus can be its first manifestation. Several adult hypothalamic–pituitary LCH patients were reported to present central diabetes insipidus as first symptom.9,10) Thus, we should have considered LCH at first time she came to our hospital.
The initial diagnosis of the pituitary stalk lesion was gangliocytoma. This tumor in sellar region is likely to be associated with pituitary adenoma.11) The histopathological diagnosis of LCH is difficult because the lesion contains varying Langerhans cells. The stage of disease at the time of biopsy may be important.8) In this case, MRI taken just before the biopsy of pituitary stalk showed that the tumor had regressed, the activity of LCH possibly decreased. We re-evaluated the first biopsy specimens of pituitary stalk after the second skull pathology showed LCH. However, Langerhans cells were not found. LCH shows the variable and multiple pathological patterns. Engelbreith-Holm et al. classified four types: The hyperplastic-proliferative phase, the granuloma phase, the xanthoma phase, and the fibrous phase.7) Langerhans cells which are positive to CD1a and S100 protein may not be detected in the tissue of fibrous phase. In view of the clinical course and histological variations of LCH,7,8) we concluded the pituitary stalk lesion was also LCH mimicking gangliocytoma. Second skull pathological examination showed classical LCH, and immunohistochemical examination showed both CD1a and S100 protein were positive. Osteolytic lesion is a major manifestation of LCH. When LCH does affect the central nervous system, it is most often found in the hypothalamus.12) A biopsy of the pituitary stalk is difficult, as biopsies of this region can cause hypopituitarism. We should carefully determine the best timing of pituitary stalk biopsy.
In 2010, oncogenic BRAF mutation was found in 57% of LCH patient samples.13) LCH is considered as an inflammatory myeloid neoplasm.14) But it has been reported that, in some cases, LCH occurs following chemotherapy, radiotherapy, and head trauma.15,16) One case of LCH occurred after burr hole surgery was reported.17) We assumed that cell activation and stimulation due to craniotomy exacerbated the LCH. In this case, the patient had not complained of back pain prior to her first biopsy. Thus, we had not examined the complete skeletal system. We assume that there had not been an osteolytic lesion before her first craniotomy. The emergence of an osteolytic lesion after her first biopsy led to a diagnosis of LCH.
In conclusion, LCH should be included as a differential diagnosis even in adult patients, especially who manifest central diabetes insipidus with hypothalamic-pituitary lesion. Besides, osteolytic lesions should be explored systemically before and after surgery in patients with suspected LCH. It is difficult to diagnose LCH histologically when the phase of disease is not active. The changes of lesion size may reflect the activity of LCH. The best timing of biopsy should be considered.
Informed consent was acquired from the patient.
The authors have no conflict of interest about this manuscript. M.K, T.H, I.S, and R.S are members of the Japan Neurosurgical Society and have registered self-reported COI Disclosure Statement Forms online.