2021 Volume 8 Issue 1 Pages 143-150
2021 Volume 8 Issue 1 Pages 143-150
Microvascular decompression (MVD) is the gold standard in the treatment of hemifacial spasm (HFS), and endovascular surgery has been described as a treatment only for aneurysm-induced HFS in several previous cases. We describe symptomatic HFS caused by a normal vertebral artery (VA) trunk adjacent to the ipsilateral dissecting VA aneurysm completely cured after stent-assisted coil embolization. A 52-year-old man presented with a 2-month history of gradually worsening left HFS. Magnetic resonance imaging (MRI) and cerebral angiography revealed a dissecting VA aneurysm on the left side. Based on the findings from preoperative MRI, not the aneurysmal dome itself, but the VA trunk just distal to the aneurysmal dome was considered likely to be compressing the root exit zone (REZ) of the facial nerve. Stent-assisted coil embolization was conducted for the VA aneurysm, and the stent was deployed to cover the wide neck of the aneurysm and offending zone of the VA trunk simultaneously. HFS started to show improvement just after the procedure and complete disappearance within 1 year. HFS was completely resolved by stenting of the offending artery. Stents may show efficacy for “intra-arterial decompression” by reducing pulsatility against the REZ of the facial nerve due to the thickness and rigidity of the stent metal and delayed endothelialization.
Hemifacial spasm (HFS) involves distressing tonic-clonic contractions of the facial muscles, caused by mechanical compression of the root exit zone (REZ) of the facial nerve.1,2) The most commonly identified etiology is ectatic or tortuous loops of normal vessels. However, tumors, arteriovenous malformations, and aneurysms are also sometimes seen as causes of this disorder.3)
Currently, microvascular decompression (MVD) remains the gold standard treatment for HFS, providing macroscopic and spatial distance between the nerve and causative vessel using a prosthesis under craniotomy.1)
We experienced a case in which an intracranial stent was placed to assist coil embolization of an unruptured vertebral artery (VA) dissecting aneurysm. As a result, concomitant HFS unexpectedly improved, since the stent covered not only the aneurysmal neck but also the responsible VA trunk at the same time. To the best of our knowledge, this represents the first report to describe complete cure of HFS by intracranial stenting of the responsible artery.
A 52-year-old man presented with a 2-month history of spasm which started from the left orbicularis muscle, spreading to the left orbicularis oris muscle. He visited our hospital for further examination and treatment. Neurological examination revealed intermittent facial spasm on the left side. Preoperative angiography and magnetic resonance imaging (MRI) including vessel wall imaging (volume isotropic turbo spin echo acquisition [VISTA]) revealed a dissecting aneurysm (8 × 10 mm) arising from the left fourth segment of the VA (Fig. 1). Rather than the aneurysm itself, the normal VA trunk just distal to the aneurysmal dome was considered likely to be compressing the REZ of the facial nerve on source images of magnetic resonance angiography (MRA) (Fig. 2). No other lesions potentially responsible for causing HFS were evident. The patient had neither taken any medication for HFS nor had willing to have MVD done. However, the patient elected to undergo treatment of the aneurysm as a potentially life-threatening pathology. Stent-assisted coil embolization was proposed, to avoid perforator injury or stump thrombosis in the setting of VA sacrifice. This study was approved by the patient consent.
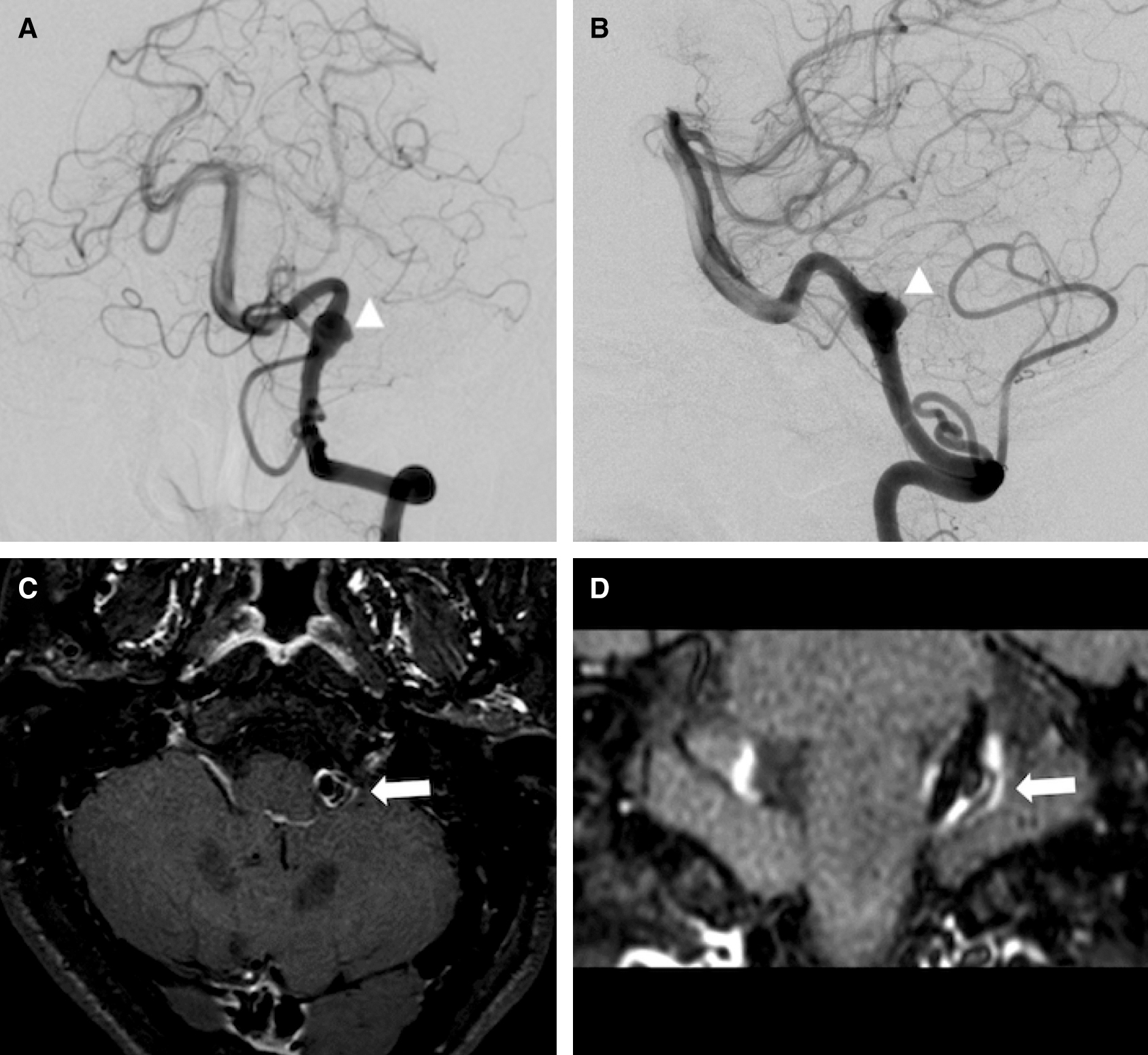
Aspirin at 100 mg/day, clopidogrel at 75 mg/day, and cilostazol at 200 mg/day were administered before the endovascular treatment.
We performed stent-assisted coil embolization using an Enterprise-2 vascular reconstruction device (VRD) 4.0 × 23 mm stent (Codman Neurovascular, Miami, FL, USA), one Orbit Galaxy complex coil (Codman & Shurtleff, Raynham, MA, USA), and nine Target 360 nano coils (Stryker, Fremont, CA, USA) under general anesthesia. The stent was successfully deployed in the parent artery to cover not only the aneurysmal neck but also the distal VA trunk as the presumptively offending area (Figs. 3A–3C).
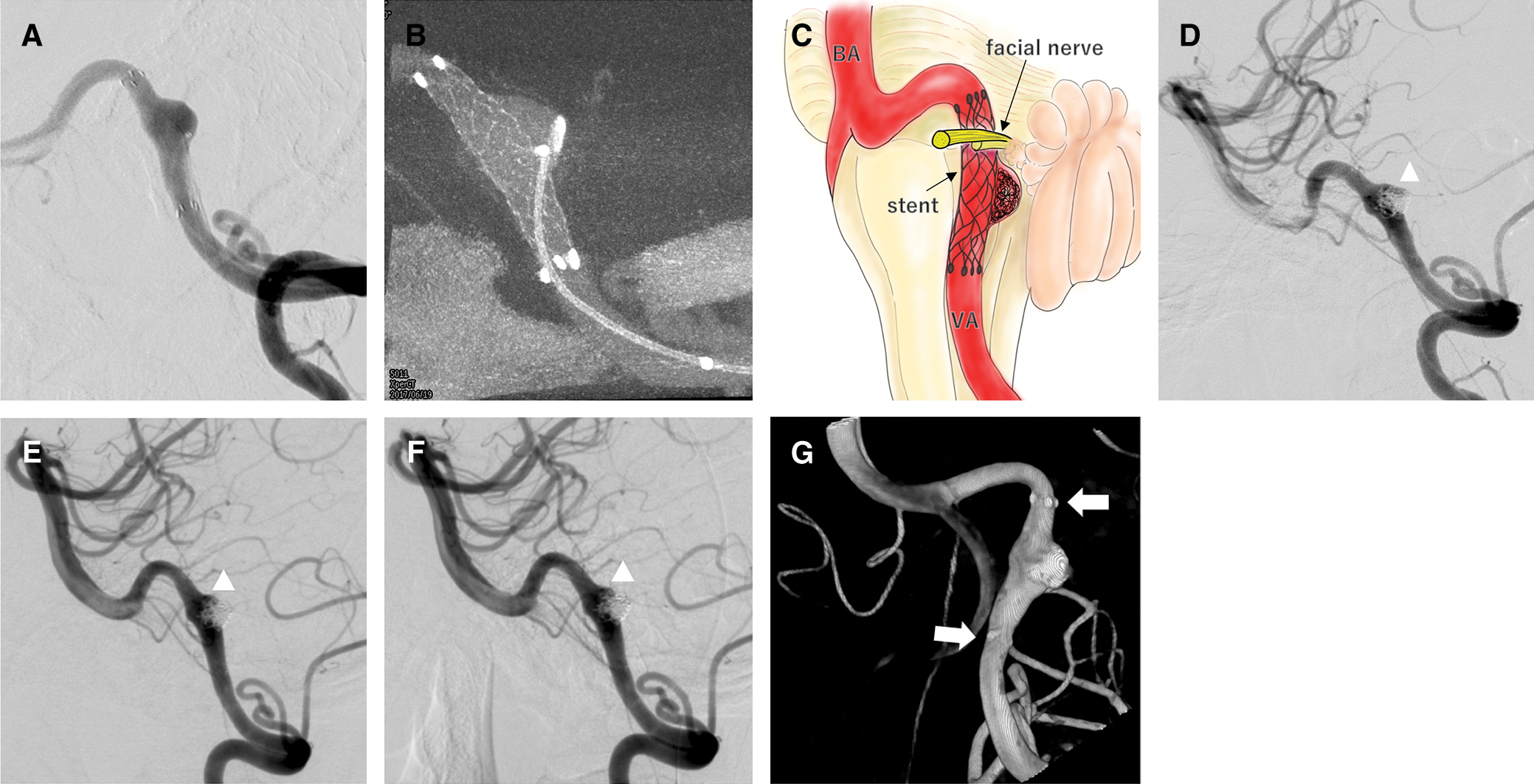
Post-embolization angiography showed sufficient obliteration, but slight contrast flow remained on the rostral side of the dome (Fig. 3D). Postoperative MRI showed asymptomatic cerebral embolism, but no other evidence of complications such as in-stent thrombosis. The patient left our hospital 4 days after embolization and clopidogrel was discontinued at this time.
Postoperative course and follow-upInterestingly, HFS started to show improvement just after the procedure and gradually decreased day by day. A reduction of approximately 50% was obtained within a month and complete resolution was obtained within 1 year. No recurrence of HFS has been noticed during 32 months of follow-up.
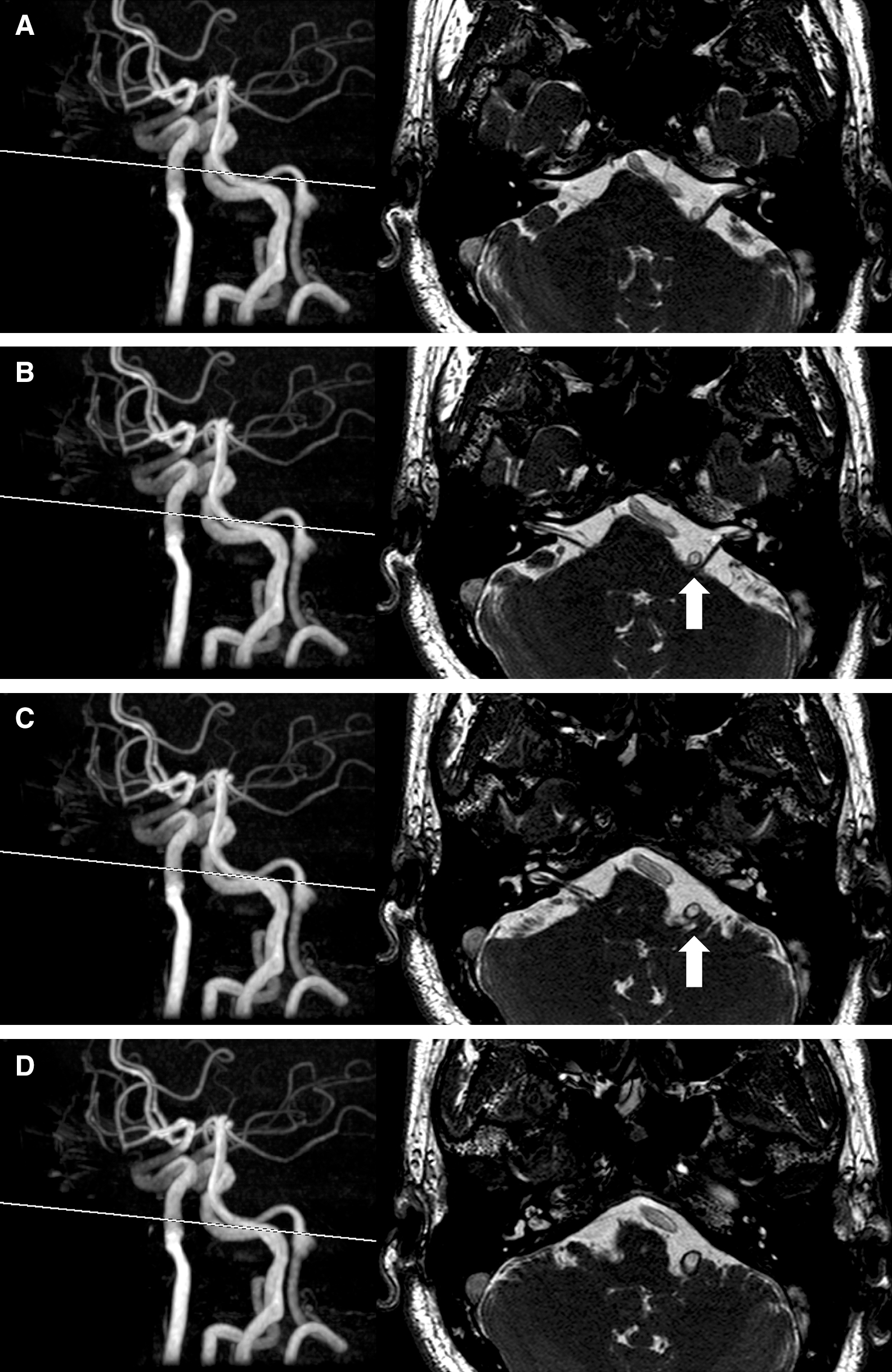
Regarding follow-up images, angiography was repeated at 14 and 26 months postoperatively. Lateral views of the left VA injection showed development of intra-aneurysmal thrombosis, but slight body filling in the distal neck remained (Figs. 3E and 3F). Three-dimensional (3D) rotational angiography at 26 months showed stent markers at both ends embedded in the VA opacification, suggesting the formation of new endothelialization on the stent surface (Fig. 3G). MRI at 10 months revealed no significant positional change between offending vessel and the REZ of cranial nerve VII compared with preoperative MRI (Figs. 2 and 4).

MVD is the current gold standard treatment for HFS. However, recent developments in endovascular techniques have contributed to the treatment of aneurysms affecting the REZ in cases where direct surgery is highly difficult. To date, at least six reports have described HFS caused by aneurysm and treated endovascularly, with successful cure achieved in each case (Table 1).4–9)
| Author | Year | Age | Offending vasculature | Sex | Type | Location | Size | Treatment | Periods from onset to treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Nagashima et al.4) | 2001 | 69 | AN | M | Dissection fusiform | VA-BA junction | N.A | Proximal occlusion | 20 months | CC after 6 months |
| Sato et al.5) | 2001 | 53 | AN | M | Dissection fusiform | VA (V4) | 8 mm | Coil trapping | More than 51 months | CC after 6 months |
| Murakami et al.6) | 2004 | 49 | AN | F | Saccular | VA (V4) | N.A | Coil trapping | More than 9 months | CC after 6 months |
| Matsumoto et al.7) | 2005 | 62 | AN | F | Saccular | VA (V4) | N.A | Coil trapping | More than 6 months | CC after 3 months |
| Nakagawa et al.8) | 2011 | 55 | AN | F | Fusiform | Near the VA union | 13 mm | Coil trapping | 50 months | CC after 3 months |
| Santiago et al.9) | 2019 | 60 | AN | F | Saccular | VA (V4) | 11 mm | Flow-diverter | 12 months | CC after 6 months |
| Ours | 2020 | 52 | Distal VA trunk | M | Dissection fusiform | VA (V4) | 10 mm | Stent-assisted coil embolization | 2 months | CC after 12 months |
AN: aneurysm, BA: basilar artery, CC: complete cure, N.A: not applicable, PICA: posterior inferior cerebellar artery, VA: vertebral artery.
Nagashima et al.4) and Santiago et al.9) reported that there were no changes in the size of aneurysm in contact with the REZ on MRI at the time freedom from symptoms was achieved, despite the disappearance of blood flow in the aneurysm due to thrombosis after endovascular surgery. In addition, endovascular devices such as stents and flow diverters reportedly reduce flow velocity in the aneurysm without changing the pressure inside the aneurysm.10) However, HFS has been improved by flow diverter placement.9) This implies that reducing pulsatility is more important for symptom improvement than decreasing compression on the REZ.
Furthermore, HFS can apparently be improved even with minute positional and hemodynamic changes in the offending vessel, according to a report regarding improvements in HFS after spinal drainage.11) Another report described selective catheterization into the causative vessel as resulting in transient improvement of HFS.12) Moreover, the arterial straightening phenomenon is known to occur frequently after intracranial stenting, and the angle of the stented artery is reported to continue to change between 6 and 34 months after stent deployment.13) In addition, the Enterprise-2 VRD, a closed-cell stent, is known to result in more prominent vessel straightening.14,15) The distal VA might move minimally after stent placement, although gross positional changes in the responsible VA area were not apparent on follow-up angiograms (Fig. 3) and MRI (Fig. 4).
In our case, it is difficult to prove pathological endothelialization inside of the stent, but important evidence suggesting new endothelial formation was the fact that markers at both ends of the stent appeared embedded in the VA opacification on 3D rotational angiography at 26 months postoperatively16,17) (Fig. 3G).
Similarly, in-stent restenosis is a well-known complication encountered 3–6 months after coronary stenting. Pathologically, this process has been attributed primarily to neointimal hyperplasia following endothelial injury due to radial force of the stent.18,19) Cerebrovascular stents not used for arteriosclerotic stenosis lesions have been suggested to cause similar changes to a relatively mild degree.20) Actually, in a recent animal experiment, Chun et al. created a nitinol-based intra-arterial neurovascular decompressor mimicking the Enterprise VRD stent in terms of stiffness and deployed it in the femoral artery of a mini-pig.21) Formation of neointima bridging over the device was observed without interrupting the patency of the arterial lumen on histopathological examination using hematoxylin and eosin staining.
Factors associated with improvement of HFS immediately after stent placement are speculated to include the stent mediating decreases in normal arterial pulsation in the causative vessel wall due to the rigidity and additional thickness of the stent metal, and possibly subtle positional changes in the causative vessel through stent-straightening effects. Endothelialization of the inner surface of the stent in response to stent implantation might then develop gradually in the chronic stage, thereby further reducing pulsatile force against the REZ day by day. This hypothetical process would seem to explain the gradual improvements in facial spasms.
Lee et al. documented that 41% of untreated HFS cured spontaneously from 2 months to 23 years in their clinical study.22) However, in our case, the patient realized significant improvement of his unpleasant symptom from just after the endovascular treatment. This fact also suggested an efficacy of our procedure rather than a natural course.
As described above, we experienced the patient with VA aneurysm treated with stent-assisted coil embolization resulting in complete cure of HFS. This unexpected result may imply an alternative treatment for HFS in case of surgical difficulty or elderly people.
HFS associated with intracranial distal VA started to improve just after the stenting of the offending artery and completely resolved within 1 year. Stents may show efficacy for “intra-arterial decompression” by reducing pulsatility against the REZ of the facial nerve due to the thickness and rigidity of the stent metal and delayed endothelialization.
We thank Ms. Naomi Tamura for her editing efforts of this manuscript.
None.