2021 Volume 8 Issue 1 Pages 79-84
2021 Volume 8 Issue 1 Pages 79-84
Pineal glial cysts associated with bilateral hearing impairment are very rare. Here, we present the case of a 13-year-old boy with a pineal cyst, which caused severe bilateral hearing impairment persisting from 6 years of age. When the patient was 6 years old, the bilateral hearing acuity was about 40 dB on audiometry. Upon admission to our otolaryngology department, his audiogram revealed a bilateral worsening of the hearing acuity (80 dB). Magnetic resonance imaging (MRI) revealed an abnormal pineal cyst with tectal compression from the left with hardly normal bilateral brainstem auditory evoked potentials (BAEPs). We obtained informed consent for exploratory surgery and employed the right occipital transtentorial approach for pineal cyst removal. Based on histological examination, we diagnosed a glial cyst of the pineal gland. At 12 months postoperatively, the patient’s hearing improved, showing a bilateral hearing acuity of 40 dB on audiometry. Since the auditory pathway has both crossed and uncrossed fibers at the upper pons and midbrain level, compression at the lateral lemniscus or inferior colliculus level can cause bilateral hearing impairment. In the present case, there was a possible slow pineal cyst growth that eventually compressed the upper pons to the midbrain, lateral lemniscuses, or inferior colliculi from the left side, this eventually led to bilateral hearing impairment. These findings indicate that surgery can improve hearing acuity in patients with a pineal cyst associated with progressive hearing impairment.
Non-neoplastic pineal cysts are relatively common incidental findings in healthy individuals. The prevalence of pineal cysts ranges from 1.1 to 4.3% in the adult population and 1.9% among children.1,2) Such cysts are typically small without clinical implications with rare symptoms, including headache, diplopia, and treatment requirement.3) In contrast, symptomatic large glial pineal cysts requiring surgical intervention without ventriculomegaly or Parinaud`s syndrome are rare.4,5) Moreover, pineal glial cysts associated with bilateral hearing impairment are very rare.6) Here, we present the case of a 13-year-old boy with a pineal cyst, which caused severe bilateral hearing impairment, without hydrocephalus or Parinaud`s syndrome, and improved postoperatively. Moreover, we discuss the possible mechanisms underlying hearing impairment, as well as the characteristics and evolution of this condition.
A 13-year-old boy presented with hearing impairment that had persisted from 6 years of age. At the age of 6 years, the patient had bilateral hearing acuity of about 40 dB on audiometry. There was a gradual decrease in his hearing acuity and his academic performance worsened sharply after the age of 10 years due to progressive hearing impairment. At this point, he was admitted to the otolaryngology department in another hospital for progressive hearing impairment where he received conservative treatment. However, the gradual hearing deterioration remained until he required a hearing aid. On admission to the otolaryngology department of our hospital, his audiogram revealed bilateral hearing acuity worsening (80 dB) (Fig. 1, left panel) compared to the previous observation, 3 months before (60 dB). Computed tomography (CT) revealed no deformity in the middle and inner ear with vestibular aqueduct expansion in the temporal bone. Regarding the bilateral brainstem auditory evoked potentials (BAEPs), there was absence of wave V, and this wave was hardly normal (Figs. 2A and 2B). Magnetic resonance imaging (MRI) revealed a pineal cyst (2.5 cm × 3.6 cm × 3.8 cm) compressing the brainstem from the left side and cerebellar surface (Figs. 3A–3C). Subsequently, the patient was referred to the Department of Neurosurgery. Neurological examination revealed no papilledema and no Parinaud’s syndrome. Moreover, there was no elevation in the levels of tumor markers, including serum human chorionic gonadotropin and alpha- fetoprotein. Pressure on the brainstem’s dorsal hearing pathway from the cystic lesion was considered as the cause of the progressive hearing impairment. We obtained informed consent for exploratory surgery and employed the right occipital transtentorial approach for pineal cyst removal. Figure 4 presents the intraoperative views. The gray-colored cyst wall was smooth and slightly thick (Fig. 4A). We fenestrated the cyst wall, which led to the release of clear fluid, and removed the posterior wall of the pineal cyst (Fig. 4B). The anterior wall of the pineal cyst was thin, and a part of the posterior wall was fenestrated (Figs. 4C and 4D). A histological examination led to the diagnosis of a pineal glial cyst (Fig. 5). There was no clear improvement of the hearing ability immediately after the operation, but hearing ability gradually improved after 3 months. At 12 months postoperatively, there was no marked improvement in the bilateral BAEPs (Figs. 2C and 2D); however, an improvement of the patient’s hearing was noted, showing a bilateral hearing acuity of 40 dB on audiometry (Fig. 1, right panel). Postoperative MRI revealed a relief of the brainstem’s compression, especially in the tectal plate and cerebellar surface (Figs. 3D–3F).
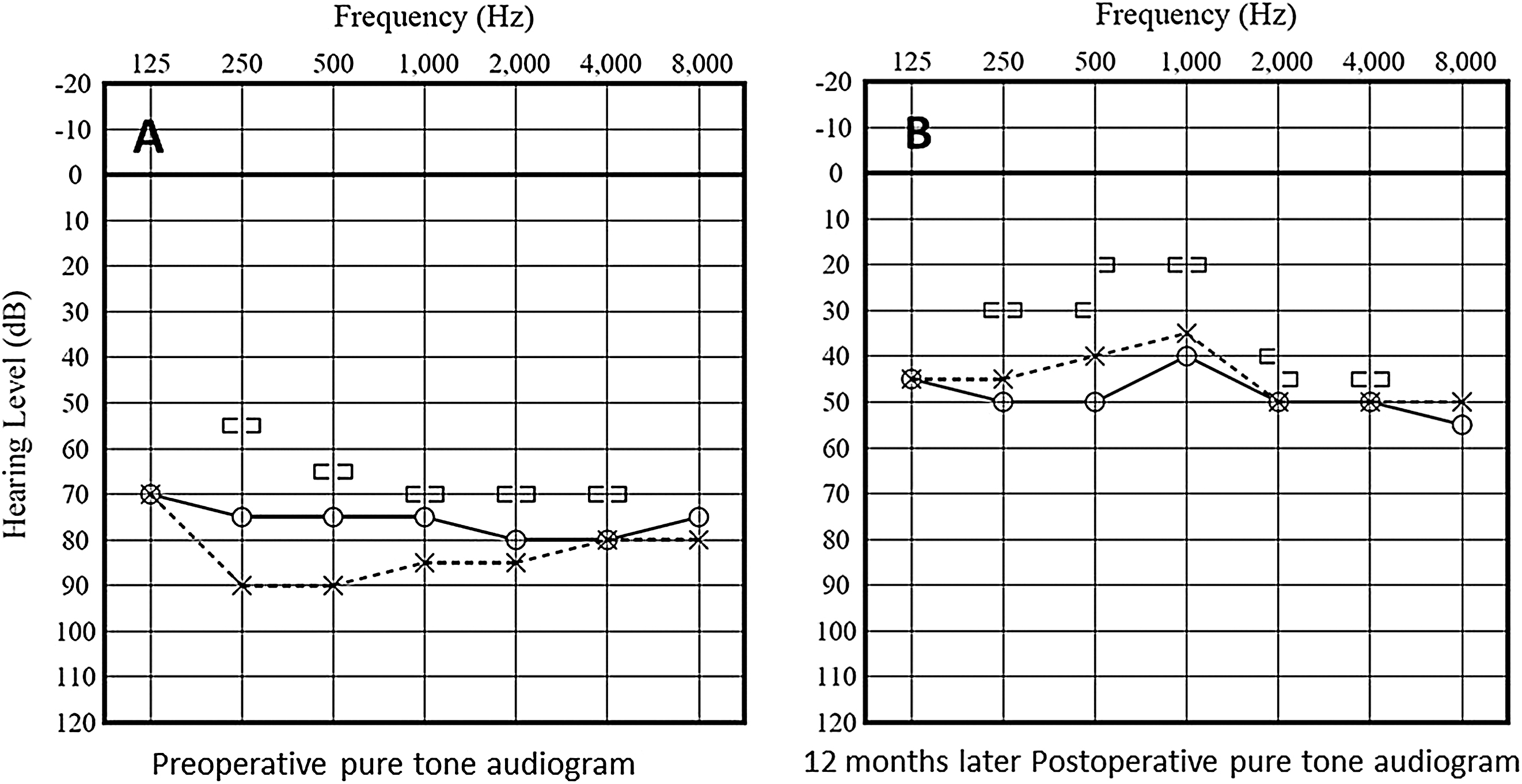




Pineal cysts are a common finding during routine autopsy and MRI, with an incidence of 21–40% and 1.4–4.3%, respectively. The peak prevalence is observed in the age group of 19–30 years, significantly decreasing with advancing age among adults. Younger age and female sex are independent factors for increased pineal cyst prevalence in adults. The pineal cyst incidence on MRI among younger patients is 1.9%.1,2,5) Compared with cysts among older patients, those in younger patients are more likely to change or grow but are likely to remain asymptomatic.1) Symptomatic pineal cysts are rare and are usually larger than 15 mm in diameter.7) Al-wolou et al. suggested that symptoms without obstructive hydrocephalus or clear signs of tectal compression should not be attributed to pineal cysts and patients should be conservatively managed.1) Contrastingly, previous studies have suggested that obstructive hydrocephalus and Parinaud`s syndrome are not required to render pineal cysts symptomatic.8,9) Our patient had a pineal cyst larger than 30 mm in diameter and presented with severe progressing bilateral hearing impairment without obstructive hydrocephalus and Parinaud’s syndrome. Moreover, there was no clear wave V in bilateral BAEPs, and it was hardly normal. BAEPs can reveal wave V loss or reduction in the case of midbrain or upper pons lesions.10) However, even under normal conditions, roughly 15–20% of all cases have the wave IV–V complex,11) which indicates that abnormalities of BAEPs do not always imply the presence of a hearing deficit12,13) and diagnosis of the cause of hearing impairment should not be solely based on BAEPs. In our case, there were no clear abnormal lesions in the middle or inner ear, and the patient exhibited progressive bilateral hearing loss. Moreover, MRI revealed a pineal cyst applying pressure to the midbrain and upper pons, and there was no clear wave V on the BAEPs. Therefore, we concluded that the progressive bilateral hearing impairment was caused by the pineal cyst.
Candidates for treatment modalities for pineal cysts are microsurgical approach, shunt surgery, and endoscopic approach. In this case, the endoscopic approach could not be used because there was no obstructive hydrocephalus caused by the pineal cyst. Moreover, it has been reported in the literature that cyst removal provides more symptom relief than shunt surgery;14) therefore, we selected an occipital transtentorial approach for microsurgical cyst removal. Hearing ability improved gradually after 3 months, and there was an improvement of the patient’s hearing on audiometry at 12 months postoperatively. In pediatric cases, reactivity to audiometry may improve with growth, but this patient was 13 years old on admission to our institution; therefore, there was little influence of reactive improvement accompanied with growth.
Common pineal tumor symptoms include headache, papilledema, and Parinaud’s syndrome, which are caused by hydrocephalus and superior colliculus compression.1) However, in our case, only bilateral hearing impairment gradually worsened. Hearing impairment is more common in pineal region meningioma than in other pineal tumors.15) The gradual growth of meningiomas might cause compression of the inferior colliculus and medial lemniscus, which causes hearing impairment without progression to hydrocephalus or Parinaud’s syndrome. Although there have been a few reported cases of meningiomas in the pineal region with hearing impairment,6) no case of bilateral hearing impairment caused by a pineal glial cyst has been reported. Pineal glial cysts are known to grow as slowly as meningiomas, unlike the rapidly growing malignant tumors, such as germ cell tumors.6,15) In preoperative MRI, not only the inferior colliculi but also superior colliculi were compressed by the pineal glial cyst, but symptoms such as double vision or Parinaud’s syndrome were not recognized. It is possible that sensory nerve fibers, such as those of the auditory pathway, were more susceptible to gradual compression than motor nerve fibers, such as those of the oculomotor nuclei; therefore, the superior and inferior colliculi were gradually compressed by the pineal region, causing only hearing impairment without Parinaud`s syndrome. In our case, there could have been slow pineal glial cyst growth, which eventually compressed the upper pons and midbrain and led to bilateral hearing impairment without Parinaud’s syndrome. The auditory pathway contains both crossed and uncrossed fibers at the upper pons and midbrain level. Therefore, unilateral compression at the inferior colliculus level could cause bilateral hearing impairment.10) In our case, MRI showed a large pineal cyst that compressed the upper pons and midbrain from the left side, which suggested compression of the lateral lemnisci and inferior colliculi and led to bilateral hearing impairment.
Although there is no clear standard timing about the operation, if the pineal cyst is considered to be the cause of the hearing impairment, operation for pineal cyst should be performed before the condition becomes a hard of hearing impairment.
The mechanisms underlying glial cyst formation and growth remain unclear. There have been several hypotheses regarding the growth of preexisting pineal cysts, including hormonal changes and hemorrhage following pineal apoplexy.16,17) Kang et al. demonstrated the communication between cysts and the third ventricle, with to-and-from cerebrospinal fluid movement, playing major roles in preexisting cyst growth.7) However, this hypothesis is inconsistent with our case, as we did not observe such communication.
Generally, pineal cysts are usually incidentally detected and remain asymptomatic without changes in size upon follow-up imaging assessments. However, for patients with pineal cysts associated with progressive hearing impairment, as in the present case, surgery could improve hearing acuity.
The authors declare that they have no competing interests.