2021 Volume 8 Issue 1 Pages 117-122
2021 Volume 8 Issue 1 Pages 117-122
Erdheim–Chester disease (ECD) is a rare systemic disease characterized by non-Langerhans histiocytosis. Pituitary involvement, often manifesting as diabetes insipidus, is the most common central nervous system (CNS) lesion. However, significant mass formation compressing the optic apparatus is rarely reported. We present a case of ECD-related suprasellar mass treated with an endoscopic transnasal approach, with emphasis on the surgical strategy and the intraoperative findings. The mass was fibrous, significantly hard, and strongly adhered to the optic nerves, causing visual impairment. A subtotal resection was performed with preserving the adhesion between the mass and the optic nerves, and her visual symptoms improved remarkably after surgery. We highlight the surgical procedure of ECD-related suprasellar mass, from an endoscopic point of view. Due to strong adhesion of the mass to the surrounding optic apparatus and perforators, complete resection may be harmful; judicious mass reduction with preserving such adhesion would contribute to better visual outcomes.
Erdheim–Chester disease (ECD) is a rare disease characterized by systemic non-Langerhans cell histiocytosis, with tissue infiltration of CD68-positive CD1a-negative lipid-laden foamy histiocytes.1,2) ECD may affect various organs, including bones, cardiovascular systems, lungs, kidney, skin, etc., potentially leading to fatal organ failure; the V600E mutation of the BRAF proto-oncogene plays a significant role in the disease progression.2–5) The involvement of the central nervous system (CNS) seen in almost half of the patients is critical because it may potentially lead to serious neurological deterioration, particularly if deep parts of the brain are involved, and is the leading cause of ECD-related deaths.6–10) CNS lesions occur throughout the neuraxis, including the intra- and extra-axial spaces, such as the hypothalamic–pituitary axis, brainstem, cerebellum, cerebrum, spinal cord, dura mater, and others.1,4,6,10–17) Diabetes insipidus is the most common symptom associated with the lesions and, in most such patients, radiographic examinations reveal an abnormal thickening of the pituitary stalk or the absence of high signal intensity in the neurohypophysis on T1-weighted images.2,17–19)
Despite its predominance in the CNS region, marked deterioration of visual function caused by the suprasellar mass is quite uncommon,20) and only a few surgical reports are available21,22); those cases underwent microsurgical resection, and detailed characteristics of the mass, including the general consistency, involvement of the surrounding critical anatomies, and the extent of adhesion, are not well disclosed. Especially for a mass involving the optic apparatus, little is known to legitimately discuss the therapeutic strategy. Here, we report a recent case of ECD with a large suprasellar lesion, in which we widely exposed the mass and the surrounding anatomies and constructed a surgical strategy to preserve the visual function.
A 65-year-old female patient had a 3-year history of bone pain in her lower limbs with bilateral pretibial erythema and her X-ray findings showed osteosclerosis on them. Simultaneously, she also had panhypopituitarism and diabetes insipidus and already had been on hormone replacement therapy. Initially, she was conservatively treated in the department of orthopedic surgery and endocrinology of our hospital. However, when she started to present with visual disturbance, including bitemporal hemianopsia and decreased visual acuity (right, 20/125 and left, 20/200), she was finally referred to our department. On magnetic resonance imaging (MRI), a heterogeneously enhanced suprasellar mass of 25 mm in diameter with peripheral calcification was detected (Figs. 1A and 1B). 18-Fluorodeoxyglucose-positron emission tomography showed an increased uptake in the joints of the extremities and bilateral tibia as well as suprasellar lesion. Based on the clinical and radiological findings, ECD was suspected. Considering the progressive visual symptoms in the patient, we decided to perform endoscopic transsphenoidal surgery (ETS) to achieve symptomatic relief, and to know the histopathology for the definitive diagnosis.
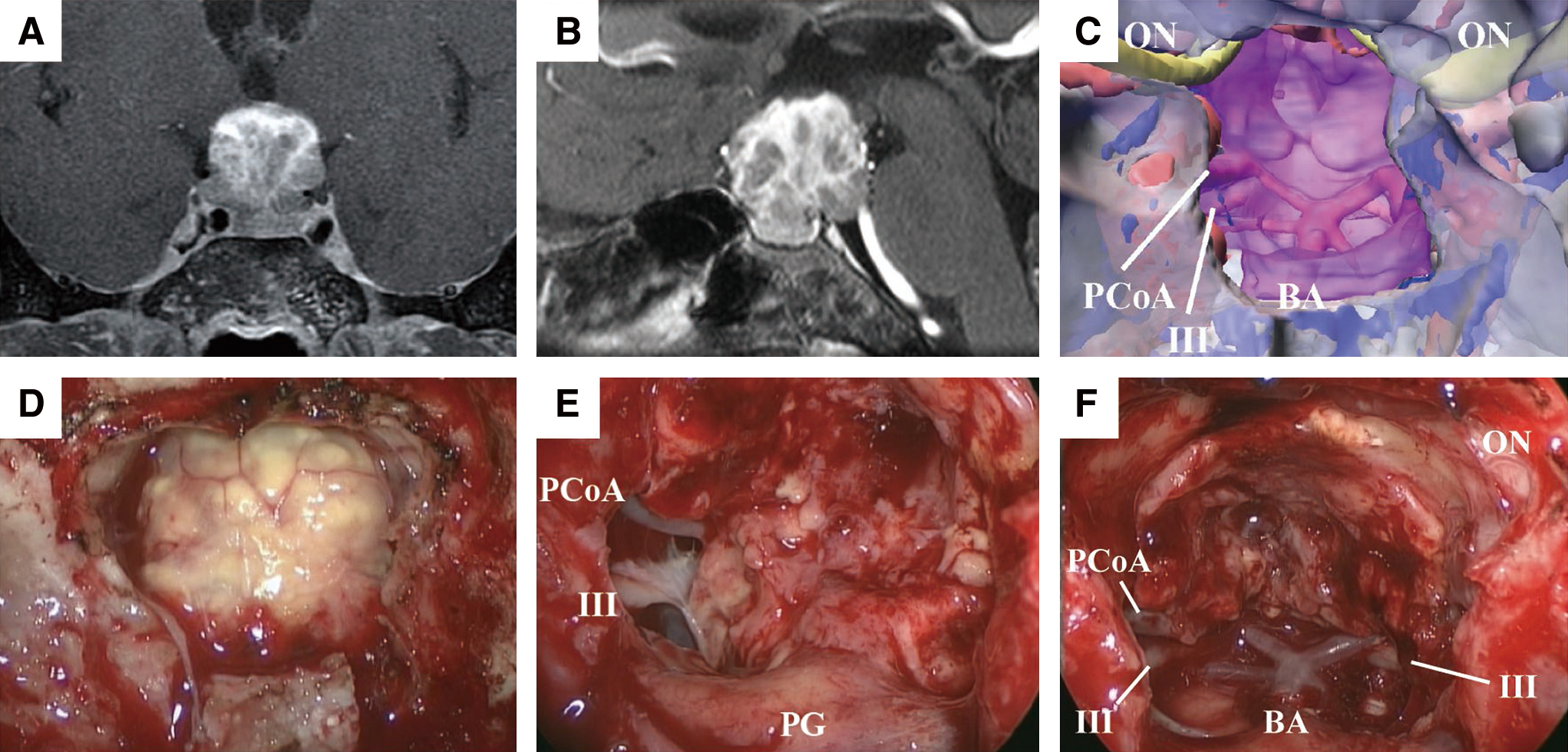
Under general anesthesia, the patient was placed in the supine position, with the patient’s head fixed in a Mayfield skull clamp. The nasal cavity was decongested with cottonoids soaked in 0.02% epinephrine. The surgical navigation system, including the intraoperative electromyogram for the extraocular muscles, and monitoring of the visual evoked potential were set up. Rigid 0- and 30-degree endoscopes (Karl Storz Endoscopy Japan, Tokyo, Japan), 4 mm in diameter and 18 cm in length, were used for the procedure. A three-dimensional reconstruction model was created based on the MRI and computed tomography for surgical simulation (Fig. 1C).
We adopted an endoscopic extended transsphenoidal approach. After removing the posterior ethmoid and lateralizing the middle turbinate bilaterally, wide sphenoidotomy was performed to expose the planum sphenoidale, the tuberculum sellae, and the sellar floor adequately. The bony structure on the sella and the planum sphenoidale were removed by drilling, and the underlining dura was opened after cauterization of the intercavernous sinus. The lesion was separated from the ventral dura mater with the thick arachnoid membrane. Following arachnoid dissection, yellowish fibrous mass was exposed (Fig. 1D), which was very hard and loosely adhered to the diaphragm sellae. However, it involved the pituitary stalk and was strongly adherent to the surrounding cerebral anatomies. We attempted sharp dissection with microscissors to debulk the mass. While the mass was not significantly vascularized, it tightly adhered to the optic chiasm, and involved the oculomotor nerves, the posterior cerebral arteries, and its perforators in the lateral aspects (Fig. 1E). Therefore, the extracapsular dissection would have involved certain risks of damaging the essential structures. Thus, we intentionally abstained from gross total resection, and achieved maximum mass reduction instead, for decompression of the optic chiasm (Fig. 1F). The patient had already lost her pituitary function and been on hormone replacement therapy before surgery; therefore, we sacrificed the pituitary stalk. After surgery, we reconstructed the skull base defect with multiple layers of fascia lata and continuous lumbar drainage was performed for the next 3 days.23) Intraoperative findings are also summarized in Supplemental digital content 1, video 1 (available Online).
Histopathological findingsHistological examination showed xanthogranulomatous foamy histiocytes with multinucleated Touton-type giant cells. Proliferation of the collagen fibers and microvasculature, coupled with lymphocyte infiltration, was detected on histopathology, suggesting that the mass was a pseudotumor. On immunohistochemical analysis, the histiocytes were CD68 positive, CD1a negative, and S100 negative (Fig. 2). The cells were positive for BRAF V600E mutation. Based on the histopathology, and the clinical and radiological findings, a diagnosis of ECD was made.1)

MRI on postoperative day 1 showed reasonable mass reduction (Fig. 3A). Her postoperative course was uneventful except for transient unilateral oculomotor palsy that completely recovered within a month. Simultaneously, her visual acuity and bitemporal hemianopsia improved remarkably over a period of 1 month (right, 20/32 and left, 20/50) and were completely resolved within 3 months. We considered starting medical treatment to target the BRAF mutation; however, it was abandoned because the medical costs related to the treatment were not covered in the national health insurance scheme. At the last follow-up, 1 year after the surgery, though MRI demonstrated mild intermittent regrowth of the residual mass, the patient remained clinically stable without steroid or interferon-α therapy (Fig. 3B).

Patient consent was appropriately gained as per our institutional protocol. Institutional Review Board approval was not sought as this is a case report and the data were collected in a retrospective way.
The optimal management for an ECD-related sellar lesion is yet to be established, owing to a scarcity of cases. While radiotherapy is reported to have only limited effect,1) long-term high-dose interferon-α may be beneficial for intractable cases.24) Moreover, given the recent findings on the association between BRAF mutation and ECD, BRAF inhibitors are expected to have a significant role in the disease management.1,25–27) However, efficacy of those medical therapies is not established yet and is still controversial whether sufficient mass reduction can be achieved. Therefore, surgical resection is currently the only available and reliable treatment modality for an ECD-related suprasellar mass.
Since the mass is non-malignant granulomatous lesion, the primary goal of surgery would favor functional preservation rather than radical resection. Nevertheless, no report exist where visual function was successfully preserved (Table 1),19,21,22,28,29) suggesting that establishing the optimal surgical strategy is important. To our knowledge, only two case reports describe intraoperative microsurgical findings as well as the surgical outcomes up until now.21,22) In those reports, the regions are described as a well-demarcated mass with firm fibrous capsule and relatively soft core, which was in line with our findings. Importantly, gross total resection through the transcranial approaches did not improve visual function, indicating that more meticulous manipulation might be needed for functional preservation. Given that the mass originated under the optic chiasm, we selected ETS to widely visualize the interface between the mass and the optic apparatus. Of note, we found that the mass was strongly adhered to the optic apparatus with involvement of the vessels supplying blood to the chiasm. The hard adhesion necessitated extremely gentle manipulation and forced us to intentionally leave a sheet of tumor on the surface of the optic apparatus aiming to restore visual impairment; indeed, visual acuity and bitemporal hemianopsia remarkably improved after surgery.
| Author year | Age sex |
Presenting symptoms | Surgery EOR |
Characteristics of mass | Adhesion | Anatomies involved | F/U period | Postoperative outcomes | Postoperative course | Final status |
|---|---|---|---|---|---|---|---|---|---|---|
| Oweity et al., 200222) | 55 M |
Hypopituitarism, DI, visual deficit | TCR GTR |
Well demarcated, soft, and yellow, supplied by ICA branches | N.D. | Opt, Inf, HT | 16 months | No improvement | No relapse, 16 months after surgery | Alive |
| Gundling et al., 200728) | 50 M |
Hypopituitarism, visual deficit | TCR N.D. |
N.D. | N.D. | Opt, Inf | 12 months | N.D. | N.D. | Alive |
| Conley et al., 201021) | 58 F |
Cognitive dysfunction, weight gain | TCR GTR |
Firm, fibrous, patchy calcification, core of the tumor was soft and grayish, moderate vascularity | N.D. | HT, 3rdV | N.D. | No improvement | (1st surgery) Deterioration in visual field, memory function, and mental status (2nd surgery) Hydrocephalus requiring shunt |
Died |
| Abla et al., 201029) | 26 F |
Hypopituitarism, DI, visual deficit | 3 TCRs STR |
N.D. | N.D. | Opt, Inf | 3.5 months | N.D. | Fractionated radiotherapy | Died |
| Sharma et al., 201319) | 52 F |
DI | N.D. Biopsy |
N.D. | N.D. | Opt, Inf | 7 years | N.D. | Slowly progressive | Alive |
| Present case | 65 F |
Hypopituitarism, visual deficit | ETS STR |
Yellowish, firm, fibrous mass with moderate vascularity | Strongly adhered to the surrounding structures | Opt, Inf, PG, PCA, CNIII, BA | 12 months | Improvement in visual function | Slowly progressive | Alive |
3rdV: third ventricle, BA: basilar artery, CN: cranial nerve, DI: diabetes insipidus, EOR: extent of resection, ETS: endoscopic transnasal surgery, F: female, F/U: follow-up, GTR: gross total resection, HT: hypothalamus, ICA: internal carotid artery, Inf: infundibulum, M: male, N.D.: not described, Opt: optic apparatus, PCA: posterior communicating artery, PG: pituitary gland, STR: subtotal resection, TCR: transcranial surgery.
Based on our case, gross total resection of large ECD-related suprasellar mass would be challenging and possibly harmful; alternatively, judicious mass reduction could ameliorate the visual dysfunction, and thus may balance necessity and safety in such cases. Wide opening of not only the anterior skull base but also the sphenoid sinus and the posterior ethmoid is undoubtedly important to obtain adequate space enabling meticulous manipulation. ETS would be greatly beneficial in clear visualization and preservation of the relationship between the mass and the surrounding critical structures while achieving safe and optimum mass reduction.
The possibility of recurrence is the downside of subtotal resection. Two previous case reports had ≥1-year follow-up; one case after gross total resection did not show regrowth over 16 months but the other after biopsy showed slow progression.19,22) Our case also showed slow progression, which would probably necessitate additional interventions at some point. However, given that the visual function of the patient has been preserved up until the last visit, judicious mass reduction through an endoscopic view would be a reasonable option. Because our report is based on a single case with a medium follow-up period, accumulating additional information through longer follow-up data and further case studies are needed to decide the best therapeutic options. Nevertheless, this paper would serve as an important piece of evidence when considering a surgical strategy for this extremely rare disorder.
None.