2021 Volume 8 Issue 1 Pages 235-240
2021 Volume 8 Issue 1 Pages 235-240
Late relapse of herpes simplex encephalitis (HSE) is defined as the recurrence of HSE more than 3 months after the initial exposure. The postoperative diagnosis of HSE following neurosurgery is complicated because the clinical presentation can mimic other common complications of neurosurgery. Cerebrospinal fluid polymerase chain reactions (CSF-PCR) is the gold standard for the diagnosis of HSE. We describe a case of late HSE relapse after epilepsy surgery in a patient who required a brain biopsy due to repeated negative CSF-PCR results. A 38-year-old woman had a history of HSE from the age of 3 years. She had intractable epilepsy from the age of 20 years and underwent right posterior quadrant disconnection (PQD) at the age of 38 years. Postoperatively, she had a right hemispheric intracerebral hemorrhage (ICH) and her consciousness was gradually worsening. Her consciousness improved after removal of the ICH. However, her consciousness gradually deteriorated again. Fluid-attenuated inversion recovery (FLAIR) revealed bilateral hyperintensity in the frontal lobes, including the white matter. CSF-PCR for herpes simplex virus (HSV) was performed twice, but yielded negative results. We performed a brain biopsy to target FLAIR hyperintensity in the right frontal lobe. PCR of the brain specimen was positive for HSV. Her consciousness improved with acyclovir, methylprednisolone, and cyclophosphamide. To our knowledge, this is a case of HSE induced by epilepsy surgery which had the longest duration until relapse after the initial HSE episode. A brain biopsy can be used to confirm the diagnosis of suspected HSE when CSF-PCR results are negative.
Herpes simplex encephalitis (HSE) is a fatal disease caused by the herpes simplex virus (HSV). Clinical features of HSE include fever, headache, seizures, and altered consciousness.1) Magnetic resonance imaging (MRI) is the most prominent imaging method for the diagnosis of HSE.2) For suspected HSE cases, intravenous acyclovir must be initiated immediately without delay to obtain definitive results.2)
Definitive diagnosis of HSE is established by a cerebrospinal fluid polymerase chain reaction (CSF-PCR), which is the gold standard for the diagnosis of HSE. CSF-PCR for HSV has a high sensitivity (>95%) and specificity (>99%)3)
Cases of HSE recurrence after epilepsy surgery have been previously reported.4–14) The postoperative diagnosis of HSE following epilepsy surgery is complicated because the clinical presentation can mimic other common complications of epilepsy surgery.
Late relapse of HSE is defined as the recurrence of HSE more than 3 months after the initial exposure. According to the literature, the longest interval of recurrent HSE was 26 years in a patient with initial exposure at under 1 year of age,14) suggesting that HSE has a long-term risk of recurrence following initial treatment.
This report presents a case of HSE recurrence 35 years after its initial exposure, which was confirmed by a brain specimen biopsy, since the CSF-PCR yielded negative results twice.
A 38-year-old right-handed woman had a history of HSE at 3 years old. She started epigastric sensation and motionless staring once a month from 20 years old. Her seizures became refractory to multiple antiepileptic medications. On the scalp electroencephalogram, interictal and ictal epileptic discharges originated from the right temporal region. Slow waves originated from the right temporo-parieto-occipital region. MRI revealed right temporo-parieto-occipital lobe atrophy (Fig. 1a). Fluorodeoxyglucose-positron emission tomography showed hypometabolism in the right temporo-parieto-occipital region. We performed a right posterior quadrant disconnection (PQD) with Sylvian dissection. There were no adhesions or cloudiness in the arachnoid membrane.
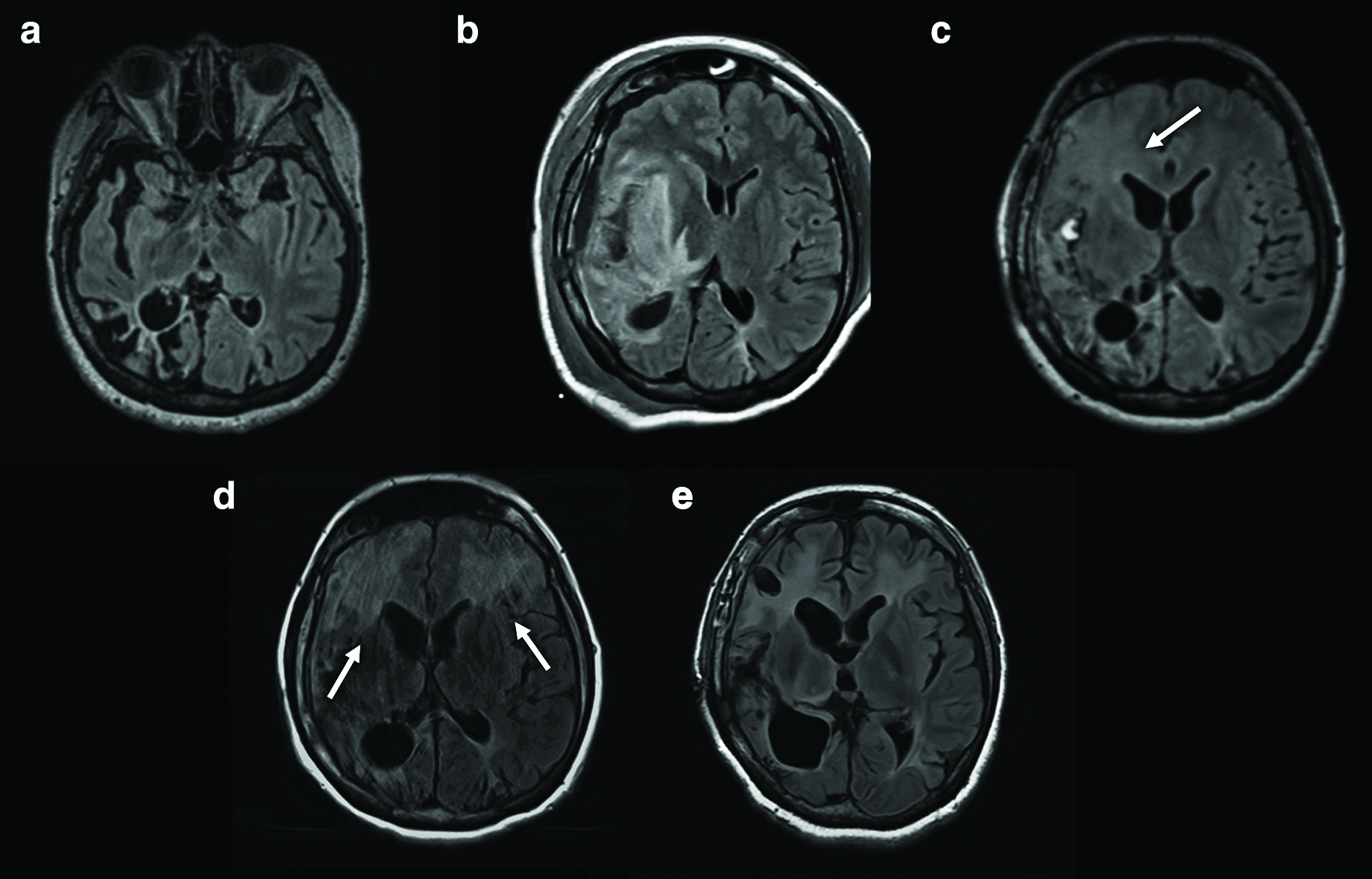
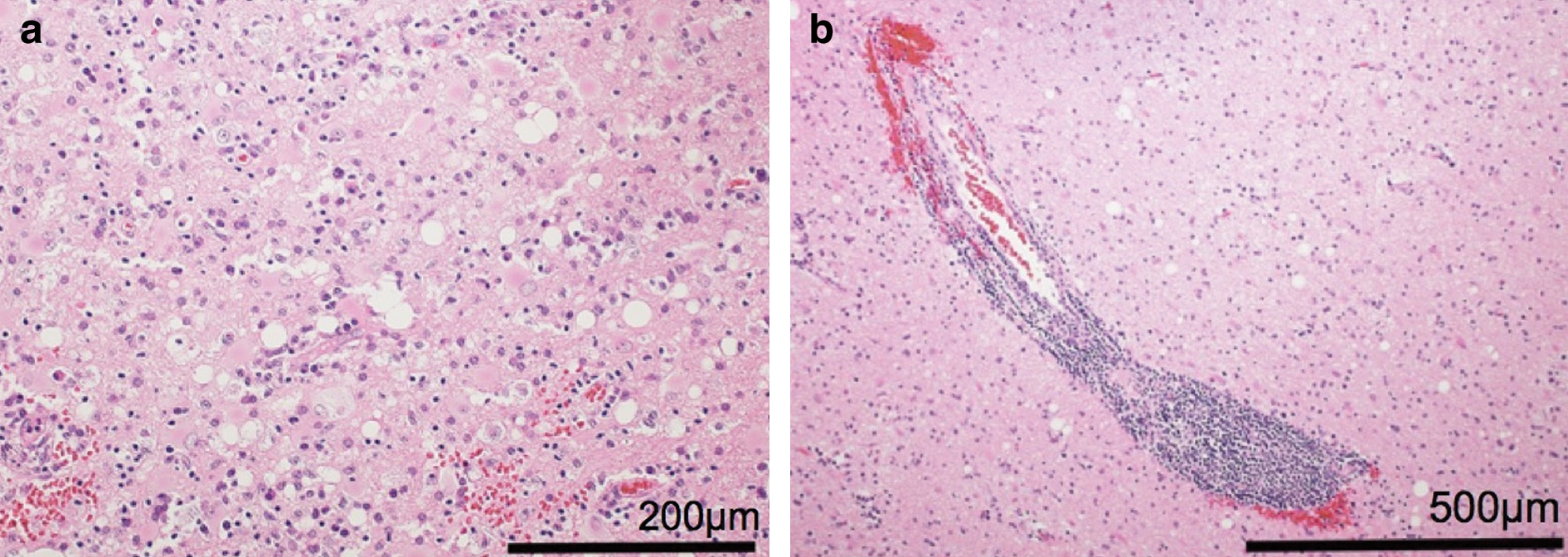
On postoperative day (POD) 1, the patient gradually developed left hemiparesis, but her consciousness was clear. The MRI demonstrated an intracerebral hemorrhage (ICH) in the right Sylvian fissure and fluid-attenuated inversion recovery (FLAIR) hyperintensity surrounding the ICH (Fig. 1b). On POD 3, her consciousness and left hemiparesis progressively worsened. Since POD 5, she had a fever of 39.0°C until POD 7. On POD 5, her white blood cell count was 8.0 × 109/L and C-reactive protein (CRP) was 8.28 mg/dL. On POD 7, her white blood cell count decreased to 7.5 × 109/L and CRP to 2.51 mg/dL. On POD 8, the FLAIR hyperintensity had spread including to the right frontal lobe (Fig. 1c) and her consciousness had worsened. We removed the ICH on POD 8. We noted that the bleeding originated in the pia mater within the Sylvian fissure, rather than the vessels. The insular cortex was slightly erosive before the evacuation of the ICH. After removal of the ICH, we confirmed brain matter pulsation. She recovered her consciousness but still had some difficulty in communicating with others. Her maximum temperature was 41.3°C on POD 9 and her fever persisted until POD 12. On POD 9, the white blood cell count was 11.1 × 109/L and CRP was 1.58 mg/dL. On POD 12, the white blood cell count decreased to 7.4 × 109/L and CRP to 1.14 mg/dL. Subsequently, she was transferred to a rehabilitation center for her hemiparesis. However, on approximately POD 40, her consciousness gradually worsened again. On POD 73, FLAIR revealed bilateral hyperintensity in the frontal lobes, including the white matter (Fig. 1d). We examined the CSF twice, on PODs 77 and 93. On POD 77, the CSF tests demonstrated the following results: 1 × 106/L cell count (all monocyte), 56 mg/dL protein and 70 mg/dL glucose. On POD 93, the CSF tests demonstrated the following results: 2 × 106/L cell count (all monocyte), 44 mg/dL protein and 69 mg/dL glucose. The real-time CSF-PCR and DNA tests were negative for HSV. To confirm the diagnosis, we performed a brain biopsy of the cortex and white matter, targeting the FLAIR hyperintensity lesion in the right frontal lobe on POD 100 (Fig. 1e). HSV-1 DNA was detected by real-time PCR of the biopsied tissue. The white matter showed vacuolar degeneration with many reactive astrocytes (Fig. 2a). These astrocytes were gemistocytic and distributed in a patchy and multifocal manner. Small vessels in the lesion were involved in perivascular lymphocyte cuffing (Fig. 2b).
Immediately after the brain biopsy, we started the patient on acyclovir (30mg/kg/day for 23 days), which failed to improve her symptoms, leading us to add intravenous methylprednisolone pulse treatment (1000 mg/day for 5 days). Because this steroid pulse therapy was only partially effective in improving her consciousness, we speculated that a strong immunosuppressant would be required for the attenuation of the inflammatory response triggered by HSV reactivation. We decided to administer cyclophosphamide (1000 mg/m2), which significantly improved her symptoms. Her consciousness and orientation were dramatically improved with mild left hemiparesis and emotional liability.
Despite the high sensitivity and specificity of CSF-PCR for HSE detection, several cases of negative CSF-PCR results in patients with HSE have been reported.15,16) The mechanism underlying false-negative CSF-PCR results might involve the following: (1) PCR analysis performed at an early stage and (2) low CSF cell counts and viral load.15,17) In the present case, we performed the CSF-PCR analysis on PODs 77 and 93, neither of which was at an early stage, revealing CSF cell counts of 1 × 106 /L and 2 × 106 /L, respectively. Therefore, brain biopsy should be considered in cases of suspected HSE and false-negative CSF-PCR result.
ICH has been reported as a complication of HSE.18) It was detected in 2.7% of 4871 patients; however, this sample did not include any postoperative cases. Common mechanisms of ICH secondary to HSE include vessel disruption, vicinity of the encephalitic process to penetrating vessels, impaired coagulation states, and the extent of the inflammatory response.19) Operative findings in our case revealed bleeding which originated from the pia mater within the Sylvian fissure, rather than from any vessels. We speculate that HSE relapse occurred immediately after PQD and impaired the coagulation states due to the inflammatory response.
A previous study on the recurrence of HSE after epilepsy surgery reported FLAIR hyperintensity, including in the temporal lobe and the atrophy to the side ipsilateral to the operation.4) In the present case, MRI on POD 1 showed an ICH along with the disconnection line, and FLAIR hyperintensity around the ICH. We assumed that altered consciousness and hemiparesis were secondary to the presence of ICH related to PQD. FLAIR hyperintensity around the ICH was likely induced by ICH-related edema. Distinguishing between recurrent HSE and other postoperative complications based on clinical features and MRI findings is challenging.
Diffuse changes to areas remote from the surgical area and captured on MRI are characteristic of HSE.20,21) Uda et al. have reported FLAIR hyperintensity in the contralateral frontal lobe, which was confirmed after epilepsy surgery in a patient with an HSE recurrence after 19 years.7) In the present case, bilateral FLAIR hyperintensity was detected in the frontal lobes on POD 73. Overall, atypical ICH after surgery, severe FLAIR hyperintensity around ICH and contralateral FLAIR hyperintensity indicated a case of an HSE relapse.
We performed a review of the literature to identify cases of relapse of HSE after epilepsy surgery (Table 1). In our review of 11 cases after epilepsy surgery, the average age of initial HSE diagnosis was 4.9 years old (ranging from 0.6 to 27 years old) and the average age at surgery was 14.5 years old (ranging from 1.9 to 29 years old). The average duration of relapse of HSE after the initial episode was 9.7 years (ranging from 1.3 to 19 years). To the best of our knowledge, the duration of relapse of HSE after the initial episode in our case was 35 years, which is the longest interval to be reported. Therefore, HSE relapse induced by an epilepsy surgery should be considered despite a long duration since the first HSE episode.
| Author & Year | History of HSE (year) | Age at surgery (year) | Surgery | Relapse of HSE (POD) | Clinical features | CSF-PCR | Acyclovir Dose/duration | Outcome |
|---|---|---|---|---|---|---|---|---|
| Lellouch et al., 2000 (case 2) | 1.3 | 8 | Left AH | 6 | Fever, seizure | Positive | UQ/3 months | Dysphasia Mild neurological deterioration |
| Gong et al., 2010 | 0.6 | 1.9 | Right hemispherotomy | 1 | Fever, irritability | Positive | 60 mg/kg/day, 3weeks | Full recovery |
| Lund et al., 2011 | 3 | 19 | Right frontal lesionectomy | 10 | Fever, seizure, headache, consciousness disturbance | Positive | UQ/UQ | Dead |
| Uda et al., 2013 | 1 | 20 | Left AH | 11 | Fever, motor aphasia, consciousness disturbance | Positive | 30 mg/kg/day, UQ | Full recovery |
| Kim et al., 2013 | 5 | 11 | Left parietal lesionectomy | 5 | Fever, seizure, lethargy | Positive | 45 mg/kg/day, UQ | Full recovery |
| Lo et al., 2015 | 6 | 17 | Right AL Right frontal disconnection |
6 | Fever, seizure | Negative | 40 mg/kg/day, UQ | Left hemiparesis Difficulty swallowing Emotional lability |
| Almedia et al., 2015 | 1.7 | 11 | Right ATL | 12 | Fever, seizure consciousness disturbance | Positive | 30 mg/kg/day, UQ | Full recovery |
| Alonso-Vanegas et al., 2016 | 2 | 10 | Right ATL | 4 | Fever, headache consciousness disturbance | Positive | UQ/UQ | Movement disorder of left hand |
| Jaques et al., 2016 (case 3) | 0.9 | 12 | Right ATL | 11 | Fever, headache consciousness disturbance | Positive | 60 mg/kg/day, 3 weeks | Worsening of preexisting left hemiplegia |
| Arnold et al., 2019 | 5 | 21 | Left ATL | 10 | Fever, seizure, aphasia | Positive | 10 mg/kg/day, 3 weeks | Single-word speech Comprehension for simple commands |
| Mantero et al., 2020 | 27 | 29 | Left ATL | 8 | Fever, seizure, confusion | Positive | 30 mg/kg/day, 3 weeks | Full recovery |
| Present case | 3 | 38 | Right PQD | 3 & >40 | Fever, consciousness disturbance | Negative | 30 mg/kg/day, 23 days | Left hemiparesis Emotional liability |
AH: amygdalohippocampectomy, ATL: anterior temporal lobectomy, CSF-PCR: cerebrospinal fluid polymerase chain reactions, HSE: herpes simplex encephalitis, POD: postoperative days, PQD: posterior quadrant disconnection, UQ: unquantifiable.
The diagnosis of HSV-1 encephalitis was established because we have detected HSV-1DNA with primers which specifically amplify HSV-1DNA. However, 23 days of intravenous acyclovir administration, the length of which is much longer than recommended for the treatment of HSV encephalitis with effectiveness of more than 99% in immunocompetent patients, did not provide any improvements for her symptoms.22–24) We did not concomitantly use any other anti-viral medications, partially because these agents have been proved to be effective for acyclovir-resistant HSV strains only at case report levels, which are mostly immunocompromised patients. Instead, methylprednisolone pulse treatment showed some benefits, suggesting that inflammatory response triggered by HSV reactivation also played a significant role. Hence, although not being a standard treatment, we tried cyclophosphamide to suppress inflammatory response.22,23)
In conclusion, our report highlights a case of long-term recurrent HSE in a patient who required brain biopsy since the CSF-PCR yielded negative results twice. All neurosurgeons need to be cautious about the potential relapse of HSE in patients with a HSE history of more than 30 years. A brain biopsy can be used to confirm the diagnosis of suspected HSE when CSF-PCR results are negative.
All co-authors confirm they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This study was approved by the ethics committee of Juntendo University (No.16-163).
The patient has consented to the submission of the case report for consideration by the journal. Verbal and written consent has been obtained from the patient for the publication of this case report and all accompanying images.
This research was supported by Japanese Science and Technology, CREST (JPMJCR 1784) and KAKENHI (19K21356). The funds are used for English proofreading and for the contribution fee.
All authors have no conflict of interest.