Abstract
Iatrogenic dissection (ID) is a well-known complication of neuroendovascular treatments. ID is predominantly attribute to endothelial injury by the manipulation of wires and/or catheters, and is generally detected in angiography during the procedure. We present a rare case with delayed ID due to deployment of a carotid stent. A 71-year-old man presented with transient motor weakness in the right extremity. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) showed previous multiple cerebral infarctions without a diffusion sign, stenosis with vulnerable plaque in the left common carotid artery (CCA), and an extremely flexed internal carotid artery (ICA). On dual antiplatelet medication, carotid artery stenting (CAS) was completed with favorable dilation of the carotid lumen. Computed tomography angiography 4 days after the procedure revealed high-grade stenosis at the ICA adjacent to the distal edge of the deployed stent. ID with intramural hematoma was diagnosed on MRI. The ID was conservatively treated and remarkably diminished 4 months after the procedure. The patient was asymptomatic during the entire clinical course. This delayed ID was considered to be due to an endothelial injury caused by the distal edge and the constant radial force of the open-cell stent against the flexed vessel and exacerbated by dual antiplatelet therapy. Even in a patient with favorable arterial dilation in CAS procedure, the possibility of a delayed ID should always be considered.
Introduction
Iatrogenic dissection (ID) is a well-known complication of cerebral angiography and neuroendovascular treatments. Generally, ID can occur due to an endothelial injury caused by manipulations of wires or catheters, and can be detected as an arterial flap or flow impairment in angiography during the procedure. Most cases of ID are asymptomatic and have a good clinical outcome. However, some ID cases could develop a cerebral infarction related to a vascular occlusion or thromboembolism.1,2) We present a rare case of delayed ID due to the deployment of a carotid stent for an atherosclerotic occlusive lesion.
Case Description
A 71-year-old man presented with transient motor weakness in the right extremity. He had hypertension controlled by medication, had received a coronary intervention for angina 1 year previously, and was taking aspirin (100 mg/day). Magnetic resonance imaging (MRI) showed the previous ischemic lesions in the bilateral cerebral hemisphere but no lesions on diffusion-weighted MRI (DWI). Time-of-flight magnetic resonance angiography (MRA) showed left common carotid artery (CCA) stenosis and an extremely flexed internal carotid artery (ICA) (Fig. 1A). Carotid duplex ultrasound demonstrated a hypoechoic lesion in the CCA stenosis and that flexed ICA was so deeply located that it was hardly detected. Carotid angiography showed 75% CCA stenosis, according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria,3) and that the ICA was bent back at approximately 85° from the carotid bifurcation, and flexed forward at approximately 130° (Fig. 2A). Black-blood T1-weighted imaging demonstrated high-intensity plaque at the CCA stenosis, with a signal intensity ratio of the plaque to the sternocleidomastoid muscle of 2.5, indicating high-grade vulnerable plaque (Fig. 1B).4) Single-photon emission computed tomography at rest showed no reduced cerebral blood flow. The patient was diagnosed as having had a transient ischemic attack due to thromboembolism from a left CCA stenosis. Following cardiological assessment, and taking into consideration the patient’s preference of therapeutic options, he was scheduled for carotid artery stenting (CAS).
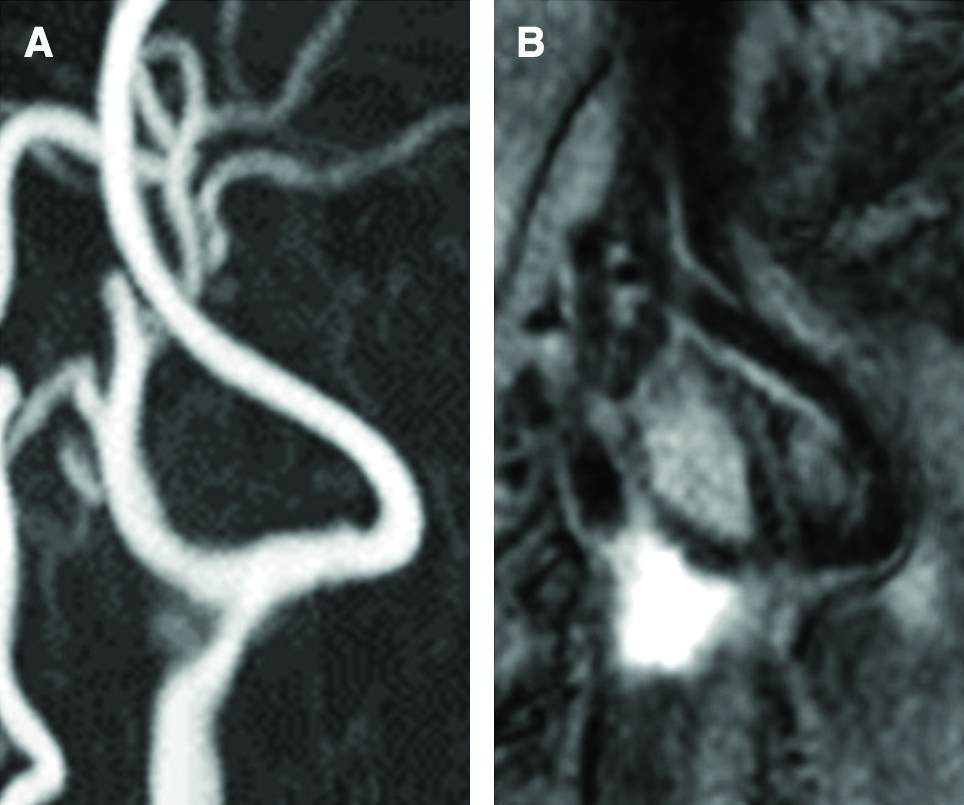
Fig. 1
(A) Preprocedural time-of-flight MRA showed the left CCA stenosis and an extremely flexed ICA without stenosis. (B) Black-blood T1-weighted imaging demonstrated high intensity at the CCA stenosis, and the signal intensity ratio of the plaque to the sternocleidomastoid muscle indicated 2.5, suggesting high-grade vulnerable plaque. CCA: common carotid artery, ICA: internal carotid artery, MRA: magnetic resonance angiography.

Fig. 2
(A) Preprocedural left carotid angiogram, demonstrating 75% stenosis of the CCA, and showed that the ICA bends backward and turns forward in acute angulations. Intraprocedural carotid angiogram findings (B–D). (B) The wire of the occlusion balloon device is positioned along the inside curve at the edge of the stent in the ICA (arrow). (C) Under the road-mapping angiography, the balloon dilation catheter was positioned exclusively at the stenotic portion of the CCA. (D) The aspiration catheter was advanced along the occlusion balloon wire, and kept along the inside curve of the ICA, away from the dissected lesion. The arrow head indicates the tip of the aspiration catheter. (E) Carotid angiogram after stenting demonstrates favorable dilation of the carotid lumen; and the ICA, distal to the stent, shows no abnormality. ICA: internal carotid artery, CCA: common carotid artery.
The patient additionally received clopidogrel (75 mg/day) orally 2 weeks before the procedure. Under local anesthesia, an 8-F long sheath-guide (Medikit, Tokyo, Japan) was placed in the left CCA proximal to the stenosis via the right radial artery. No catheters or wires were cannulated to ICA for navigation of the sheath guide. Systemic heparinization was achieved with target activated clotting times between 300 and 350 sec during the procedure. Common carotid angiography revealed that the diameters of mid-CCA, stenotic site, proximal, and distal ICA were 7.6, 2.0, 5.9, and 3.5mm, respectively. At first, a distal occlusion balloon protection device (Carotid GuardWire PS; Medtronic, Santa Rosa, CA, USA) was placed in the ICA distal to the extremely flexed portion. A 3.0 mm × 40 mm balloon dilation catheter was placed in the stenotic segment of the CCA and inflated after intravenous injection of 0.5 mg atropine sulfate to avoid hypotension with the distal balloon as protection. Thereafter, a 10.0 mm × 40 mm carotid open-cell stent (PRESICE; Cardinal Health Japan, Tokyo, Japan) was deployed from the ICA to the CCA, fully covering the stenosis (Fig. 2B). Subsequently, a 6.0 mm × 20 mm balloon dilation catheter was positioned at the stenotic portion of the CCA and inflated for 30 seconds at 6 atm (Fig. 2C). An aspiration catheter was advanced along the occlusive balloon wire and along the inside curve of the ICA (Fig. 2D). Approximately 100 mL of blood was aspirated to retrieve the debris. Finally, the protecting distal occlusion balloon was deflated and retrieved. No thrombus formation or plaque protrusion was angiographically observed during the procedure. The final angiogram demonstrated, though slight enlargement of ICA diameter due to distal occlusion balloon, favorable dilation of the carotid lumen without any abnormality and distal embolism in the cerebral circulation (Fig. 2E). In closing, after the devices were removed, the puncture site was manually compressed to perform hemostasis.
DWI on the following procedural day revealed three ischemic spots in the left cerebral hemisphere, but no neurological deficit was observed. Computed tomography angiography 4 days after the procedure revealed ICA stenosis with well-circumscribed margins and no infiltration of contrast media, indicating 77% in the NASCET criteria, at the outside curve of the ICA adjacent to the distal edge of the carotid stent (Fig. 3A). Time-of-flight MRA showed slight hyperintensity at the ICA stenotic lesion (Fig. 3B), and the CCA was invisible due to an artifact caused by the metal stent. Black-blood MRI showed high intensity at the ICA stenosis (Fig. 3C). These findings suggested intramural hematoma due to arterial dissection after the deployment of a carotid stent.5) Although transcarotid and -oral duplex ultrasonography was attempted to demonstrate the lesion in detail, it was impossible because it was located extremely deeply. Dual-antiplatelet medication was continued, and repeated DWI 5 days after the procedure showed no new ischemic cerebral lesion. The carotid stenosis spontaneously improved to 60% on computed tomography angiography 11 days after the procedure (Fig. 3D). The patient was discharged without any neurological deficits 12 days after the procedure. Computed tomography angiography 4 months after the procedure revealed that the ICA stenosis was additionally dilated and the intramural hematoma remarkably diminished (Fig. 3E).

Fig. 3
(A) Computed tomography angiography 4 days after the procedure shows 77% ICA stenosis with well-circumscribed margins and no infiltration of contrast media, at the outside curve of the ICA adjacent to the distal edge of the carotid stent. (B) Time-of-flight MRA 4 days after the procedure showed a slight hyperintensity at the ICA stenotic lesion (arrow), and the CCA was invisible due to the artifact caused by the metal stent. (C) Black-blood T1-weighted imaging showed high intensity at the ICA stenosis, suggesting an intramural hematoma (arrow head). (D) The ICA stenosis improved to 60% on computed tomography angiography 11 days after the procedure. (E) Carotid computed tomography angiography 4 months after the procedure showed improved ICA stenosis and a remarkably diminished intramural hematoma. ICA: internal carotid artery, CCA: common carotid artery.
Discussion
ID of the carotid and vertebral arteries at cervical segments are well-known complications which can occur during the diagnosis of cerebral angiograms and neuroendovascular treatments. The overall incidence of IDs as complications of these procedures varies between 0.26% and 0.7%.1,6) However, limited in endovascular mechanical thrombectomy for acute ischemic stroke, IDs could occur in 1.0%–3.9% of all the patients.7,8) There could be an elevated risk of ID during mechanical thrombectomy because of emergency, complex manipulations (e.g., coaxial microcatheter control, catheter exchanges), and the necessity for more distal placement of wide-diameter catheters even to elongated vessels, and the unexpected patients’ movement.9) There have been a few reports of evidence relating IDs to CAS procedures. Some researchers reported IDs occurred in 0.7%–3.6% of CAS patients.10,11) Cremonesi et al. revealed three patients with IDs caused by over-inflation of distal occlusion balloons.10) Tolva et al.12) reported a case with ID at ICA, caused by injury due to passage of filter guidewire. Gungoren et al.13) alone reported a case of delayed ID at CCA, presenting slight neck pain 2 weeks after CAS, caused by over-inflation of the proximal balloon of Mo.Ma device.
We presented a rare case of a delayed ID due to the deployment of a carotid stent. The preprocedural MRA revealed no plaque at the postprocedural dissected area. Moreover, final angiography showed favorable patency of the CCA and the ICA without any findings of arterial dissections. Thus, the clinical course and postprocedural findings in computed tomography angiographies and MRIs indicated a delayed intramural hematoma due to an arterial dissection, not restenosis, plaque protrusion, and/or thrombus formation. Although the existence of a tear in the arterial wall is unknown because carotid duplex ultrasonography could not detect such due to its deep location, intramural hematoma without an arterial wall tear in the aorta was previously reported and accurately elucidated.14)
The ID in the present case was considered to have been caused by the stent deployment, because the dissection started adjacent to the distal edge of stent. Moreover, the mechanism underlying the arterial dissection could have been an endothelial injury due to the deployment of an open-cell stent. Every metallic stent should be preferably positioned to a straight vessel. However, in the present case, the distal edge of the stent was positioned into the extremely flexed vessel so the stent struts might have injured the endothelia of the ICA. Moreover, it has been reported that the radial force of the open-cell stents is relatively strong, compared with that of closed-cell stents.15) To avoid vessel wall injury due to the edge of stent, it should be preferably positioned parallel to the vessel wall. In the present case, position of the distal edge of stent should be placed more proximal to the flexed portion of the ICA and so that the stenotic site at the CCA was adequately covered.
A few reports previously showed that the over-inflation of occlusion balloon was a major cause of ID. In the present case, inflation of distal occlusion balloon might result in retrograde ID. However, final angiograms showed, though slight enlargement of ICA diameter, no vessel wall injury. Thus, there is less likelihood of a cause of ID. The other causes of endothelial injury including manipulation of the occlusion balloon wire, dilation balloon, and thrombus aspiration catheters could also be completely undeniable. However, as these catheters were coaxially advanced along the distal balloon wire and straighten the flexed ICA, the inside curve of the ICA might have been injured. Anyhow, postprocedural cone-beam CT might be helpful to determine the detailed morphological analysis of arterial wall.
In the present case, the mechanisms of the delayed occurrence were thought to be caused by the constant radial force of the stent. Maynar et al. researched the change in the stent diameter following carotid stenting without using balloon angioplasty. They showed that the diameter of the stent gradually expands, especially up to 3 months after the procedure.16) Also in the present case, the constant radial force of the stent could, over time, have injured the endothelia, because angioplasty for the ICA was not performed in this CAS procedure. Additionally, dual antiplatelet medication likely contributed to the delayed enlargement of the intramural hematoma.
Neurological complications due to ID develop in less than 3% of all cases of ID.17) Although a standard treatment for IDs has not been established or reported in the literature, Paramasivam et al.1) recommended a short course of heparin in the acute phase followed by a several-month course of antiplatelet therapy. However, stenting could be feasible for patients with poor cerebral circulation, size increase of a pseudoaneurysm, a greater than 70% degree of luminal stenosis, and/or failure of medical therapy. These treatment strategies may induce complications in fewer than 3% of all the patients with IDs.1,2) In the present case, as repeated DWI and CTA showed no new cerebral infarction and spontaneous dilation of stenotic site, additional anticoagulation therapy and carotid stenting were not performed. However, heparinization should be considered in cases with new cerebral infarction or flap formation of arterial wall, even if without progression of the stenosis. As early detection and appropriate management could result in an excellent outcome, careful attention should be paid to avoid missing asymptomatic arterial dissections using non-invasive neuroimaging, for example, computed tomography angiography or MRA, even if an arterial dissection is angiographically invisible during the procedure.
Conclusions
We reported a rare case of a delayed ID due to the deployment of a carotid stent. The delayed ID was considered to be due to endothelial injury caused by the distal edge and the constant radial force of an open-cell stent to an extremely flexed vessel and exacerbated by the dual antiplatelet therapy. Even in a patient with favorable arterial dilation after CAS, the possibility of a delayed ID should always be considered.
Acknowledgments
We thank Robert E. Brandt, the Founder, CEO and CME, of MedEd Japan, for editing and formatting the manuscript.
Conflicts of Interest Disclosure
The authors report no conflicts of interest (COI) concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1) Paramasivam S, Leesch W, Fifi J, Ortiz R, Niimi Y, Berenstein A: Iatrogenic dissection during neurointerventional procedures: a retrospective analysis. J Neurointerv Surg 4: 331–335, 2012
- 2) Groves AP, Kansagra AP, Cross DT, Moran CJ, Derdeyn CP: Acute management and outcomes of iatrogenic dissections during cerebral angiography. J Neurointerv Surg 9: 499–501, 2017
- 3) North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, et al.: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445–453, 1991
- 4) Yamada K, Yoshimura S, Kawasaki M, et al.: Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis 215: 399–404, 2010
- 5) Yao B, Yang L, Wang G, et al.: Diffusion measurement of intraplaque hemorrhage and intramural hematoma using diffusion weighted MRI at 3T in cervical artery. Eur Radiol 26: 3737–3743, 2016
- 6) Cloft HJ, Jensen ME, Kallmes DF, Dion JE: Arterial dissections complicating cerebral angiography and cerebrovascular interventions. AJNR Am J Neuroradiol 21: 541–545, 2000
- 7) Pereira VM, Gralla J, Davalos A, et al.: Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke 44: 2802–2807, 2013
- 8) Jovin TG, Chamorro A, Cobo E, et al.: Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372: 2296–2306, 2015
- 9) Goeggel Simonetti B, Hulliger J, Mathier E, et al.: Iatrogenic vessel dissection in endovascular treatment of acute ischemic stroke. Clin Neuroradiol 29: 143–151, 2019
- 10) Cremonesi A, Manetti R, Setacci F, Setacci C, Castriota F: Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke 34: 1936–1941, 2003
- 11) Hong JH, Kang J, Yeo MJ, et al.: The 10-year trend of periprocedural complication following carotid artery stenting; single center experience. Cardiovasc Intervent Radiol 38: 280–287, 2015
- 12) Tolva V, Bertoni GB, Bianchi PG, Keller GC, Casana R: Immediate surgery for acute internal carotid artery dissection and thrombosis during filter deployment prior to stenting: a case report. Vascular 21: 247–250, 2013
- 13) Gungoren F, Besli F, Tanriverdi Z, Kocaturk O, Tascanov MB: Unusual complication of carotid artery stenting as the result of a proximal emboli protection device (the Mo.Ma): Iatrogenic common carotid artery dissection. Anatol J Cardiol 22: 203–206, 2019
- 14) Vilacosta I, San Román JA, Ferreirós J, et al.: Natural history and serial morphology of aortic intramural hematoma: a novel variant of aortic dissection. Am Heart J 134: 495–507, 1997
- 15) Voûte MT, Hendriks JM, van Laanen JH, et al.: Radial force measurements in carotid stents: influence of stent design and length of the lesion. J Vasc Interv Radiol 22: 661–666, 2011
- 16) Maynar M, Baldi S, Rostagno R, et al.: Carotid stenting without use of balloon angioplasty and distal protection devices: preliminary experience in 100 cases. AJNR Am J Neuroradiol 28: 1378–1383, 2007
- 17) Borota L, Mahmoud E, Nyberg C: Neuroform Atlas stent in treatment of iatrogenic dissections of extracranial internal carotid and vertebral arteries: a single-centre experience. Interv Neuroradiol 25: 390–396, 2019



