2021 Volume 8 Issue 1 Pages 295-300
2021 Volume 8 Issue 1 Pages 295-300
Intravenous indocyanine green (ICG) videoangiography is reportedly useful for vascular neurosurgery, and for treating hemangioblastoma due to its high vascularity. Videoangiography obtained after intra-arterial ICG injection has emerged as a more useful option than that after intravenous injection. This report offers the first description of a case of hemangioblastoma successfully treated using intra-arterial ICG videoangiography, and describes the efficacy of this technique. A 20-year-old man presented with progressive cerebellar ataxia and dysphagia. Magnetic resonance imaging (MRI) revealed an enhanced solid tumor in the medulla oblongata. Digital subtraction angiography (DSA) showed a highly vascularized tumor. Surgery was performed to remove the tumor in a hybrid operating room. A catheter was introduced into the vertebral artery (VA) for intra-arterial ICG videoangiography. Superficial feeders and drainers were identified and flow dynamic changes in the tumor were assessed by intra-arterial ICG videoangiography. The tumor was removed after confirming lack of flow in the drainer. Intra-arterial ICG videoangiography was more useful than intravenous ICG videoangiography in hemangioblastoma surgery for identifying feeders and drainers and assessing flow dynamics in the tumor. Use of Flow 800 made these findings simpler and easier to evaluate.
Hemangioblastomas are benign tumors that occur mainly in the posterior fossa and spine, and complete resection results in favorable outcomes. However, this tumor remains surgically challenging because the highly vascular, arteriovenous malformation (AVM)-like character necessitates en-bloc tumor removal.1–3) A good understanding of the feeding and draining vasculature and assessment of flow dynamics in the tumor are thus crucial during surgery.
Indocyanine green (ICG) videoangiography is frequently used in vascular neurosurgery due to the integration of equipment for this modality into operative microscopes. Previous reports have shown the efficacy of ICG videoangiography for cerebral aneurysm, extracranial-intracranial bypass, and cerebral AVM.4–9) Since ICG videoangiography has been confirmed as useful for identifying feeders, niduses, and drainers and for assessing flow dynamics of the nidus in AVM surgery,4,5,8) this technique has also been applied to hemangioblastoma surgery and favorable results have been reported.10–12) However, all such studies have used intravenously injected ICG. In cases of intravenous ICG videoangiography, ICG remains in the vessels during the late phase, representing an obstacle to understanding the vascular anatomy and time-dependent flow dynamic changes. In addition, the long washout time required to remove the ICG means that ICG videoangiography cannot be repeated within a short period of time. A few recent studies have examined intra-arterial injection of ICG, describing its usefulness for cerebral aneurysms and AVMs by overcoming the technical limitations of intravenous ICG videoangiography.13–15) Here, we provide the first description of hemangioblastoma treated successfully using intra-arterial ICG videoangiography during surgery in a hybrid operating room and the efficacy of this technique.
A 20-year-old man presented with progressive cerebellar ataxia and dysphagia. Magnetic resonance imaging (MRI) revealed an enhancing solid tumor with a maximum diameter of 3.0 cm accompanying a cyst in the dorsal medulla oblongata (Fig. 1A). Digital subtraction angiography (DSA) demonstrated a highly vascularized tumor mainly fed by bilateral posterior inferior cerebellar arteries (PICAs) and radiculomeningeal arteries from bilateral C2 segmental arteries (Figs. 1C and 1D). The patient underwent surgical resection of the tumor under a preoperative diagnosis of hemangioblastoma. We considered intraoperative assessment of flow dynamics in the tumor as necessary to safely remove this highly vascularized tumor in an en-bloc manner as in AVM surgery. Intra-arterial ICG videoangiography is routinely performed in the hybrid operating room (Fig. 1E) for AVM surgery in our institution to assess the flow dynamics of niduses using an ICG videoangiography technology-integrated microscope (Kinevo 900; Carl Zeiss, Oberkochen, Germany). Intra-arterial ICG videoangiography was approved by the ethics review board at Tokushima University and the patient provided written informed consent. This surgery was therefore performed in accordance with our routine methods for AVM surgery.
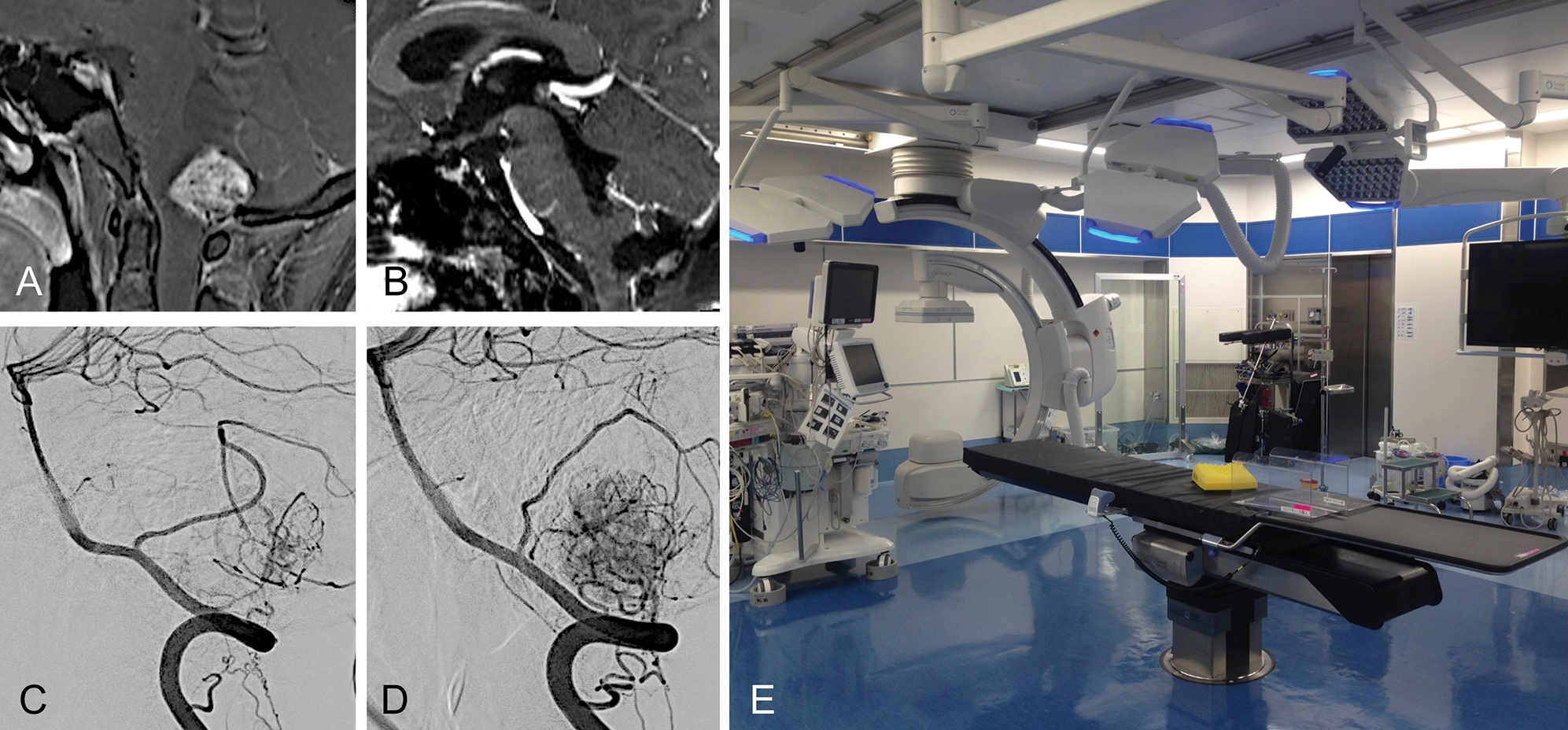
After inducing general anesthesia, the patient was positioned prone. A 4-Fr sheath was inserted into the right and left radial arteries. A heparin-coated 4-Fr catheter was then introduced into the right and left vertebral arteries (VAs). Midline suboccipital craniotomy and C1 laminectomy were then performed. After opening the dura mater, multiple tumor-related vessels were exposed in the cisterna magna (Fig. 2A). Intra-arterial ICG videoangiography was performed before tumor dissection by injecting ICG through catheters placed in both VAs, and most superficial feeders in the surgical field demonstrated on ICG videoangiography were from the tonsillohemispheric branch of the right PICA. We therefore mainly used the catheter placed in the right VA for intra-arterial injection of ICG. The videoangiography showed superficial feeders appearing at 1 s, after which the feeders disappeared. The drainers appeared at 5 s, then disappeared at 10 s. Normal veins were still present at 10 s (Figs. 2B–2D). ICG was washed out from all vessels within approximately 15 s. After coagulating the superficial feeders of the caudal and right sides (Fig. 2E), ICG videoangiography disclosed superficial feeders on the left side at 1 s, whereas some drainers were still present at 10 s, indicating reduced flow in the tumor (Figs. 2F–2H). After dissection of the superficial part of the tumor (Fig. 2I), ICG videoangiography demonstrated no appearance of superficial feeders at 1 s, whereas drainers were still present at 5 and 10 s, indicating that the tumor was supplied by the remaining deep feeders (Figs. 2J–2L). The deep part of the tumor was carefully dissected by coagulating the deep feeders (Fig. 2M). ICG videoangiography subsequently showed that the main drainer was still filled with ICG (Fig. 2N), indicating that the tumor had not been completely dissected. The tumor was removed after ICG videoangiography showed no filling of ICG in the main drainer (Fig. 2O and 2P). The captured video was analyzed using Flow 800. Prior to dissection (Fig. 3A), the color of the drainer was yellow (Fig. 3B), changing to blue after partial coagulation of the superficial feeders on the caudal and right sides (Figs. 3C and 3D). This demonstrated that Flow 800 can detect even small decelerations in blood flow. As seen in a diagram for region of interest (ROI)-1 (Figs. 3E and 3F), a slower increase was seen compared to that on the pre-dissection diagram. In addition, compared to the pre-dissection diagram (Fig. 3E), the delay time in ROI-1 was prolonged, and blood flow slowed after partial coagulation of the superficial feeders (Fig. 3F).


The catheter was left in the same position until total dissection of the tumor was confirmed. To avoid thromboembolic complications, pressurized (280 mmHg) 500-mL bags of heparinized saline (4 U/mL) were connected to the catheters for the purpose of continuous flushing, with manual catheter irrigation performed every 15 min. Although 3 mL of ICG at a concentration of 2.5 mg/mL is usually injected for intravenous use, 3 mL of ICG at a concentration of 0.05 mg/mL was injected through the catheter for intra-arterial ICG videoangiography.
Pathological examination confirmed hemangioblastoma. Postoperative MRI revealed total resection of the tumor (Fig. 1B). The postoperative course was uneventful, and the patient was discharged with slight ataxia and clumsiness of the left hand.
This report demonstrated that intra-arterial ICG videoangiography was useful for identifying superficial feeders and drainers of hemangioblastoma due to the brighter, higher phase contrast, as compared to that for conventional intravenous ICG videoangiography. In addition, washout time was shortened to approximately 15 s from all vessels after intra-arterial injection and 15 min after intravenous injection,5) enabling repeated studies within a short period to confirm reduced or complete lack of filling of the drainers. We performed videoangiography eight times in this surgery. This allowed quick comparison of multiple videoangiography studies due to the short span, and easy recognition of changes in flow dynamics for the tumor. Flow 800 also provided objective data such as delay time and speed, which helped confirm reduced flow in the hemangioblastoma.
Hemangioblastomas are highly vascular tumors with AVM-like characteristics. Strategies based on AVM surgery are thus necessary for safe removal of this tumor.1–3) Since previous studies have reported the efficacy of intraoperative ICG videoangiography for AVM surgery,4,5,8) intravenous ICG videoangiography has been applied to hemangioblastoma surgery and confirmed as useful.10–12) We provide here a first report of hemangioblastoma surgery using intra-arterial injection of ICG, with descriptions of the advantages of the method, better contrast views and shorter ICG clearance time.
Intra-arterial ICG videoangiography contributed to the differentiation of feeders and drainers of the tumor due to the brighter and higher contrast images. In addition, the rapid clearance of ICG allowed repeated videoangiography studies for the review of changes in flow dynamics of the tumor and to confirm the absence of ICG flow in the main drainer before tumor removal. Although introducing a catheter is time-consuming and carries some risk of thromboembolic complications, inserting a catheter into the VA via the radial artery is not particularly difficult with the patient in a prone position and we have not encountered any adverse coagulation events in AVM surgery thanks to the implementation of countermeasures. The pros and cons of intra-arterial and intravenous ICG videoangiography are summarized in Table 1. In accordance with this table, more complicated and vascularized angioarchitectures of the tumor make better candidates for intra-arterial ICG videoangiography to make the surgery much safer.
| Pros | Cons | |
|---|---|---|
| Intra-arterial ICG videoangiography | ✓ High contrast ✓ Easy to understand complex vascular anatomy ✓ Short washout time ✓ Easy to check flow dynamics frequently |
✓ Time-consuming setup ✓ Risk of thromboembolic complications |
| Intravenous ICG videoangiography | ✓ Easy setup ✓ Low risk of complication |
✓ Low contrast ✓ Difficult to understand complex vascular anatomy ✓ Long washout time ✓ Difficult to check flow dynamics frequently |
ICG: indocyanine green.
The disadvantages of both intravenous and intra-arterial injection of ICG videoangiography are that neither can detect feeders or drainers covered by brain tissue. All the feeding and draining vessels can be visualized by intraoperative DSA, but not by ICG videoangiography. Although we did not need to perform intraoperative DSA during or after resection of the tumor in this case, since the main drainer was found to be superficial and confirmed by ICG videoangiography, preparing for intraoperative DSA in a hybrid operating room was necessary in case of a need to compensate for ICG videoangiography. However, DSA images are inferior to ICG images with regard to the simplicity, speed, and spatial resolution of the surgical view, as ICG videoangiography can detect vessels in the same plane as the operative microscopic view. Surgeons can thus easily and rapidly detect the vasculature around the tumor without interrupting the surgical procedure.
Although intra-arterial injection of ICG was not mentioned in the FDA approval, injection of tiny amounts of ICG as compared to that used during intravenous injection should be acceptable. More cases need to be accumulated to confirm the validity and safety of this technique.
We have reported a case of hemangioblastoma that was successfully treated using intra-arterial ICG videoangiography. This technique was more useful than intravenous ICG videoangiography for identifying feeders and drainers and for assessing tumor flow dynamics. Flow 800 increased the ability to more easily understand the resulting information.
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication. All authors who are members of The Japan Neurosurgical Society (JNS) have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.