2021 Volume 8 Issue 1 Pages 287-293
2021 Volume 8 Issue 1 Pages 287-293
Dermatofibrosarcoma protuberans (DFSP) originates from the dermal layer of the skin; the optimum treatment is an extended marginal resection. We describe a case of DFSP of the scalp with a skull invasive defect that was thoroughly examined pathologically to determine the optimum length of surgical margins. The tumor cells infiltrated up to 26 mm into the dermal tissues, whereas no infiltrating tumor cells were present in the skull, indicating the combination of marginal resection of the dermal tissues and lower of the skull can be a clinically relevant strategy for treatment of DFSP cases with skull invasion.
Dermatofibrosarcoma protuberans (DFSP), a rare soft tissue neoplasm, originates from the dermal layer of the skin. The main etiological factor in the development of DFSP is the presentation of several prior traumas, including surgical and trauma scars, burns, and insect bites.1) DFSP usually occurs in young to middle-aged patients but can present in all age groups.2) It is commonly found on the trunk; however, it can also develop in the extremities, head or neck. DFSP demonstrates local infiltrative growth but seldom metastasizes distally.3) The diagnosis and optimum treatment of DFSP are usually by pathological examinations and extended resection, respectively. The mainstay of DFSP treatment is surgical, with tumor size and anatomic site being recognized as important factors influencing choice of adjuvant radiation.4) Radiation is usually reserved for large or recurrent DFSP tumors and is most effective in reducing the rate of recurrence after surgery on large tumors.5) In addition, adjuvant radiation therapy is most often used in cases where resection is limited due to anatomic site of the head and neck. In conventional extended resection surgeries, a 30-mm dermal resection margin is recommended.6) On the other hand, compared to DFSP in the trunk or extremities, DFSP in the head has extremely high local recurrent rates, ranging from 50% to 75%.7) However, few reports referring to the resection margin of skull invasion exist.
In this report, we present a case of DFSP of the scalp with skull defect that was thoroughly examined pathologically to know the optimum length of surgical margins, especially in the cases with skull invasion.
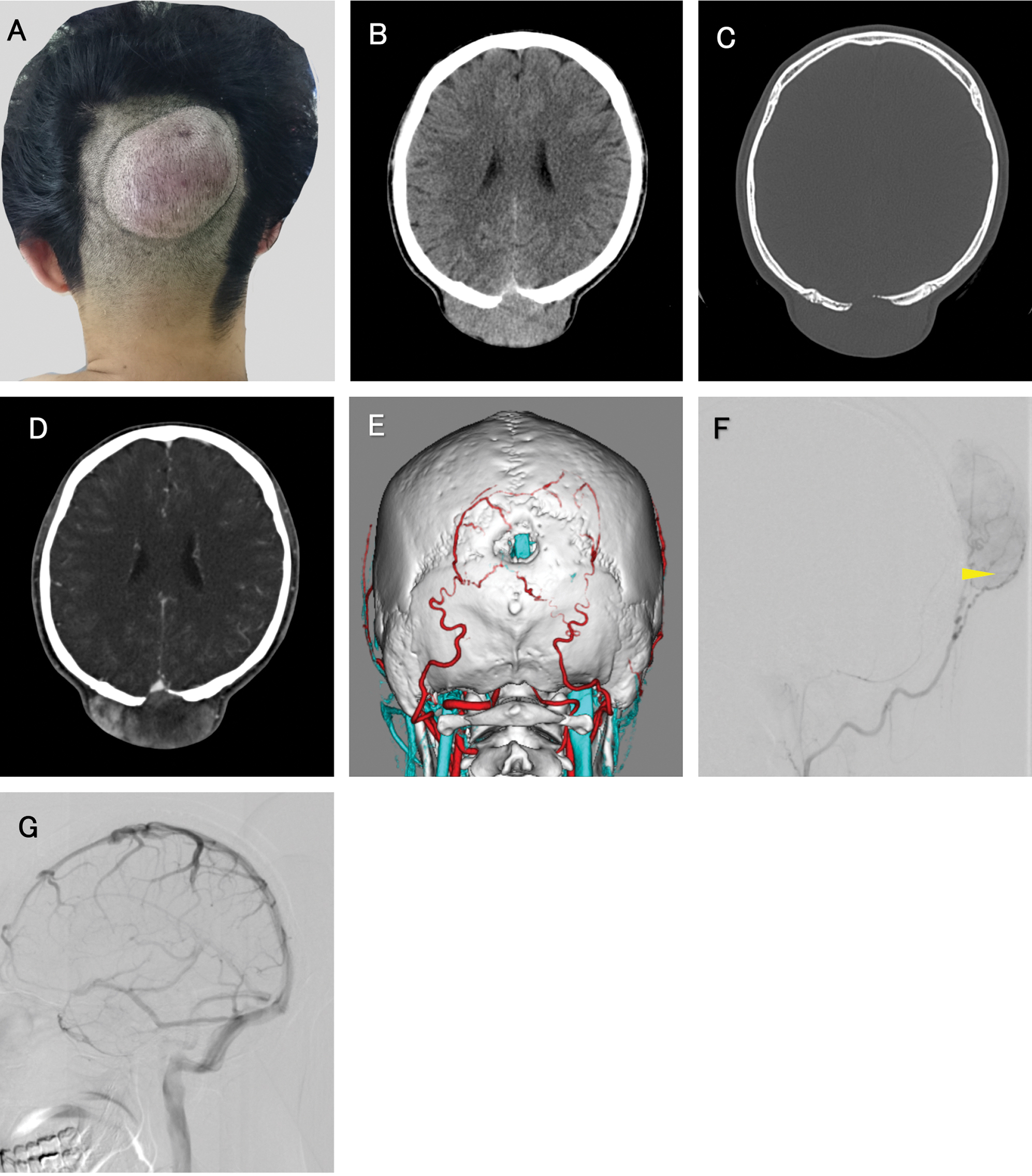
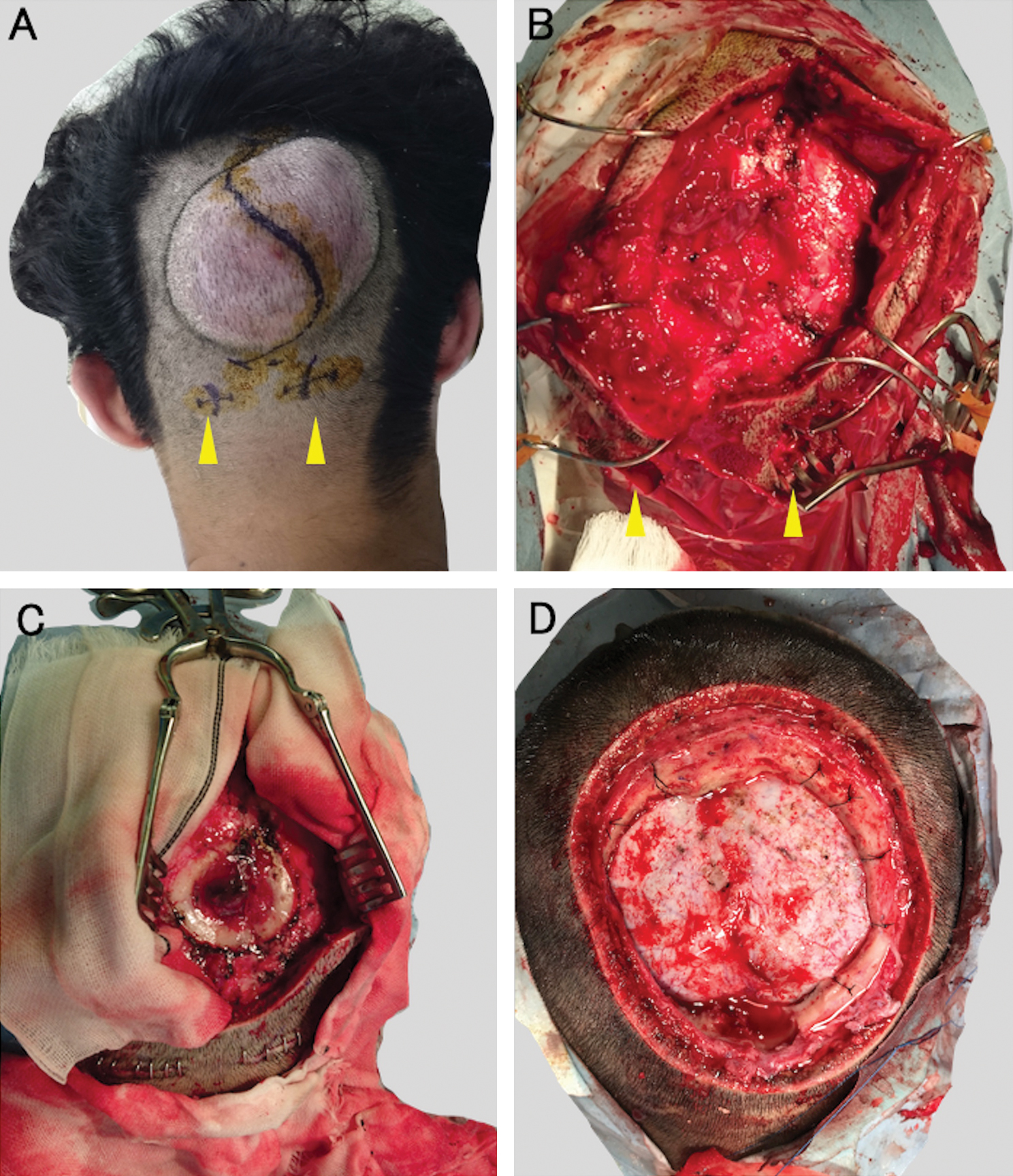
A 24-year-old male presented with a subcutaneous mass on the occipital region of his head, which he noticed after a blunt injury occurred 12 months earlier. He described that the mass only recently started increasing prior to his visit to the clinic. Upon physical examination, an oval elastic soft mass was observed in the subcutaneous layer of the occipital region (Fig. 1A). Computed tomography (CT) of the head revealed that a 15-mm diameter round skull defect was located just beneath the mass (Figs. 1B and 1C) and demonstrated heterogeneous enhancement of the subcutaneous mass (Fig. 1D). In three-dimensional CT imaging, the superior sagittal sinus (SSS) ran underneath the skull defect (Fig. 1E). The selective occipital artery angiogram revealed the development of feeding arteries around the mass (Fig. 1F). And there was no connection between superior sigmoid sinus and tumor (Fig. 1G). Laboratory analysis on his blood sample revealed no abnormalities in coagulation or hematological values. First, an atypical growing hematoma was suspected, and the mass was surgically removed (Fig. 2A). The tumor was hemorrhagic with a heterogeneous red color; the boundary was unclear with normal dermal tissues. Before the removal procedure, bilateral occipital arteries were ligated to control bleeding (Fig. 2B) and the mass was mostly resected (Fig. 2C). Artificial bone was applied to the skull defect, and the surface was formed smoothly. A pathological examination on the tissues revealed the presence of DFSP and positive surgical margins. As a result, the patient was scheduled for radical resection as the optimum surgical treatment. A contrast-enhanced magnetic resonance imaging (MRI) of the head was performed before the second operation; the dura mater beneath the skull defect was thickened and enhanced reactively, but no brain invasions were observed. A month later with pathological diagnosis of DFSP, in the second operation, the tumor was resected by wide excision with 30 mm margins with the local skin, subcutaneous tissue, and periosteum removed together (Fig. 2D). The cranial bone was also retracted with a 20 mm margin from the skull defect edge. Though dura matter beneath the skull defect was slightly discolored (Fig. 1B), normal color dura tissue was identified with scraping off the thickened surface. Skin and skull defects were reconstructed using a flap from the latissimus dorsi muscle. After the second surgery, the patient has been stable up to 14 months without recurrence.
In the pathological examination, the tumor sample resected showed proliferations of monomorphic spindle cells of DFSP with a high nucleus/cytoplasm (N/C) ratio and Ki-67 labeling index (Fig. 3A). Immunohistochemical (IHC) analysis showed diffusely positive expressions of CD34 (Fig. 3B). DFSP is characterized by formation of the collagen type I-a1/platelet-derived growth factor-b (COL1A1–PDGFB) fusion gene resulting in the constitutive upregulation of PDGFB expression.8) In this case, fluorescence in situ hybridization (FISH) using a dual-color break-apart probe of PDGFB demonstrated split signals with amplification, which indicated an unbalanced translocation covering the region (Fig. 3C).

The skin and skull resected during the second operation were multiply sliced and were subjected to thorough pathological examination. The surgical margin of the resected skin was pathologically negative (Fig. 3D). The tumor cells infiltrated up to 26 mm (range: 0–26 mm) from the macroscopic tumor edge in subcutaneous tissues. In multi-sliced subcutaneous specimen, the invasion front of tumor cells showed continuity from the main mass without isolated tumor cells. While no infiltration tumor cells were observed in both cortical and cancellous bones, even around the edges of the region of the skull with defects (Fig. 3E). Together, we confirmed pathologically negative findings in the surgical margins of the skin and cranial bone of this patient.
DFSP is characterized by a low metastasis and high growth rates, which may determine high levels of local invasion recurrence. Some reports indicate that conventional surgery leads to local recurrence in up to 30% of cases, and compared to DFSPs in the trunk or extremities, DFSPs in the head region have extremely high, ranging from 50% to 75% local recurrence rates.7–9) There is no report mentioning to the extent of infiltration of DFSP tumor cell into the skull pathologically. A radical skull resection for recurrent DFSP in the scalp was reported to have shown low clinical local recurrent rates with marginal resection of 20 mm in the cranial bone and we performed the same distant margins and analyzed the pathological infiltration to the skull in this case.10) In our case, no tumor cells infiltrating bone tissues around the skull defect edge were present in detailed pathological analysis. We suppose that the difference in the invasiveness into normal tissues is due to tumor origin and affinity, as is noted in other malignant tumors.11)
We also performed PubMed search for accessing the MEDLINE database using the terms “dermatofibrosarcoma protuberans” and “scalp” to review the surgical strategies for DFSP patients in the scalp with periosteum, skull, or intracranial invasion. In all, 74 articles were collected on DFSP of the scalp, and we reviewed articles with descriptions about periosteum, skull, and intracranial invasion. Tumor extension from the scalp into the intracranial space is very rare, and to the best of our knowledge, only 17 cases have been reported, which fulfilled the conditions above. Clinical data are summarized in Table 1 with our case.10–26) According to the review, dermal resection with periosteum was an accepted strategy with good local control if the tumor invasion was limited within periosteum. In the four reported DFSP patients whose invasion were limited within periosteum, there were no recurrence in the follow-up periods (range: 4–156 months). There were five DFSP cases with follow-up results whose invasion were limited within skull. Four patients among the five cases got surgery with marginal resection of the skull, which showed better local control comparing to a patient with no marginal resection of the skull (she developed another recurrence in 3 months after the surgery).
| Study | Age (year), sex | Primary/reccurent | Single/ multiple | Tumor size (mm) | Skin margin (mm) | Pericranium/ skull/ intracranial invasion | Skull margin (mm) | PFS, follow-up (month) |
|---|---|---|---|---|---|---|---|---|
| Rockley et al., 1989 | 23, F | P | S | 80×110 | 30 | Yes (periosteum invasion) | Periosteum resection | 22 (no reccurence) |
| Loss et al., 2005 | 26, F | R | S | 10×8 | 25–30 | Yes (periosteum invasion) | Periosteum resection | 156 (no reccurence) |
| 33, F | P | S | 50×10 | Mohs | Yes (periosteum invasion) | Periosteum resection | 4 (no reccurence) | |
| Arifin et al., 2014 | 26, M | R | S | 70×60×50 | 20 | Yes (periosteum invasion) | Periosteum resection | 12 (no reccurence) |
| McLoughlin et al., 1992 | 76, F | R | S | 5 | 30 | Yes (skull invasion) | No marginal resection | ND |
| Sinha et al., 2001 | 45, M | R | S | ND | 30 | Yes (skull invasion) | 30 | 4 (no reccurence) |
| Kim et al., 2007 | 30, F | R | S | 60×40×60 | 20 | Yes (skull invasion) | 20 | 24 (reccurence) |
| Bhatnagar et al., 2013 | 35, M | R | S | 80×80 | 50 | Yes (skull invasion) | 30 | 6 (no reccurence) |
| Liansheng et al., 2014 | 26, F | R | ND | 90×90 | No marginal resection (patient’s demand) | Yes (skull invasion) | No marginal resection | 3 (reccurence) |
| Faried et al., 2017 | 29, M | R | S | ND | 40 | Yes (skull invasion) | At least 30 | ND |
| 49, M | R | S | ND | 40 | Yes (skull invasion) | At least 30 | ND | |
| Present case | 24, M | P | S | 80 × 80 | 30 | Yes (skull invasion) | 20 | 12 (no reccurence) |
| Burkhardt et al., 1966 | 35, M | R | M | ND | Not operated | Yes (intracranial invasion) | Not operated | Within 36 (death) |
| Rockley et al., 1989 | 35, F | R | M | 70×60×30 | ND | Yes (intracranial invasion) | ND | Intraoperative death |
| Das et al., 2000 | 40, M | R | S | 100×100 | 30 | Yes (intracranial invasion) | ND | ND |
| Taniguch et al., 2002 | 39, M | R | M | ND | No marginal resection | Yes (intracranial invasion) | No marginal resection | 12 (death) |
| Uematsu et al., 2003 | 49, M | R | S | ND | No marginal resection | Yes (intracranial invasion) | No marginal resection | 5 (reccurence), 66 (death) |
| Marakovic et al., 2008 | 41, M | R | S | ND | ND | Yes (intracranial invasion) | Resected “margin around the infiltrated region” | 8 (reccurence) |
| Abe et al., 2009 | 22, M | R | S | 80×80×70 | ND | Yes (intracranial invasion) | ND | 18 (reccurence), 145 (death) |
| Sangrador et al., 2019 | 25, F | P | S | 125×105×80 | ND | Yes (intracranial invasion) | No marginal resection | ND |
DFSP: dermatofibrosarcoma protuberans, M: multiple, Mohs: Mohs micrographic surgery, ND: no data, P: primary, R: recurrent, S: single
Most cases in the review were recurrent tumor cases, so we considered why only a few cases with skull invasion were reported at initial presentation. According to the reports, the main reason seemed that DFSP was not anticipated and surgical resection was initially performed without margin for the mass, which resulted in recurrence of the local area. And when the patients recurred, they are often introduced to specific institutes for advanced medical service.
This case report is the first to analyze the extent of invasion of DFSP tumor cells into the skull pathologically. These findings indicate that the combination of extended resection of the dermal and lower margins of the skull can be a clinically relevant strategy for treatment of DFSP cases in the head with skull defect, which is clinically significant and has the potential to inform strategies that may possibly reduce surgical invasiveness.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional with the Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the individual participant included in the study.
This study was partially funded by KAKENHI Grants-in-Aid for Scientific Research, [18K16565 (T.N.)] and received no specific grant from any funding agency in commercial or not-for-profit sectors.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.