2021 Volume 8 Issue 1 Pages 315-318
2021 Volume 8 Issue 1 Pages 315-318
Hemorrhagic venous infarction secondary to deep brain stimulation (DBS) surgery occurs rarely and can cause delayed intracranial hemorrhage. Venous cerebral infarction after DBS surgery is commonly caused by coagulation of the superficial cerebral veins, which usually produces transient symptoms but leaves no permanent sequelae. We report a case of hemorrhagic venous infarction resulting in severe sequelae, likely due to coagulation of the lateral venous lacuna during DBS surgery.
Deep brain stimulation (DBS) surgery is performed routinely for patients with movement or neuropsychiatric disorders. There are risks associated with DBS surgery, with one of the most serious complications being intracranial hemorrhage. Delayed intracranial hemorrhage can result from the rare occurrence of hemorrhagic venous infarction secondary to DBS surgery.1) Generally, hemorrhagic venous infarction and concomitant regional brain edema derive from iatrogenic occlusion of a surface vein of a substantial caliber. We describe a case of massive hemorrhagic venous infarction secondary to DBS surgery and resulting specifically from occlusion of a lateral venous lacuna.
A 71-year-old, right-handed man with a 10-year history of Parkinson’s disease (PD) was referred to our department for DBS surgery. Aside from the PD, his medical history was unremarkable, and he had never taken anticoagulant drugs. Preoperative laboratory tests confirmed the absence of coagulopathy. There was no family history of a movement disorder or of cerebrovascular disease.
Before being referred to us, several types of anti-PD drugs had been administered, but the patient’s symptoms gradually worsened, affecting his activities of daily living. He finally chose to undergo DBS surgery. The main purpose of the DBS was to improve the uncontrolled wearing-off phenomenon and levodopa-induced dyskinesia.
Prior to surgery, we created planning of the tentative target for STN and electrode trajectory on magnetic resonance (MR) images of a 3D T1-weighted magnetic image and a transverse magnetic susceptibility-weighted fast field-echo image sequences (vein BOLD; veins are clearly visible in these images) using the dedicated software StealthStation (Medtronic, Minnesota, MN, USA). The tentative target was set on the dorsolateral portion of subthalamic nucleus (STN) based on the coordinate of the anterior commissure- posterior commissure (AC-PC) line and the hypo intensity region observed on susceptibility-weighted fast field-echo images, and the entry point was determined so that the trajectory did not pass through vessels in the brain, the sulcus, or the lateral ventricle, and penetrates the STN as long as possible (Fig. 1).

Under general anesthesia, a stereotactic Leksell frame was secured to the patient’s head. With the frame in place, computed tomography (CT) images of the brain were obtained. The images were transferred to the StealthStation system, where they were fused with the prescan MR images. After fusion of the images, the target coordinates were calculated on the system. The target coordinates were as follows: 12.0 mm lateral to, 1.52 mm posterior to, 4.52 ventral to the mid-point of the AC-PC line. Surgery began with placement of a burr hole approximately 2.5 cm left of the midline and 2.2 cm anterior to the coronal suture, according to the surgical plan. After incision of the superficial layer of the dura mater, we observed, between the superficial layer and deep layer, a relatively large pool of venous blood covered with a thin film. We coagulated and cut the offending intradural vessel to achieve hemostasis. We did not find a major cortical vein under the burr hole, and thus there was no need for us to sacrifice any surface cortical vein to insert the electrodes. Extracellular multi-unit recordings were obtained by means of three microelectrodes, and on the basis of the microrecording data, the DBS electrode was inserted. Intraoperative C-arm X-ray was used to verify that the tip of the electrode was situated at the center of the target area. The DBS lead implantation surgery took approximately 2 hours. That same day, an implantable pulse generator (Activa SC; Medtronic) was placed in a subcutaneous pocket created on the patient’s left anterior chest wall, also under general anesthesia.
The patient’s status was monitored peri-operatively. His blood pressure remained stable, and there were no remarkable changes in other vital signs. Immediately following the procedure, he was neurologically intact. Fourteen hours after surgery, however, he lost consciousness. CT revealed a 10 × 6.5-cm irregular subcortical hemorrhage, pronounced cerebral edema, and a 2-cm left-to-right midline shift (Fig. 2). We immediately evacuated the intracerebral hematoma. Craniotomy exposed the incised dura matter just beneath the DBS burr hole, but there were no occluded bridging veins adjacent to the DBS burr hole. Intraoperative indocyanine green (ICG) video angiography showed a delay in cerebral venous circulation as well as late-phase retrograde venous drainage via a superficial cerebral vein (Fig. 3). ICG angiography also revealed another venous lacuna just posterior to the DBS burr hole, and a small cortical vein emptied directly into this lacuna (Fig. 3). Unfortunately, the patient did not recover from the deep coma after evacuation of the hematoma and the external decompression; rather, he fell into a persistent vegetative state.
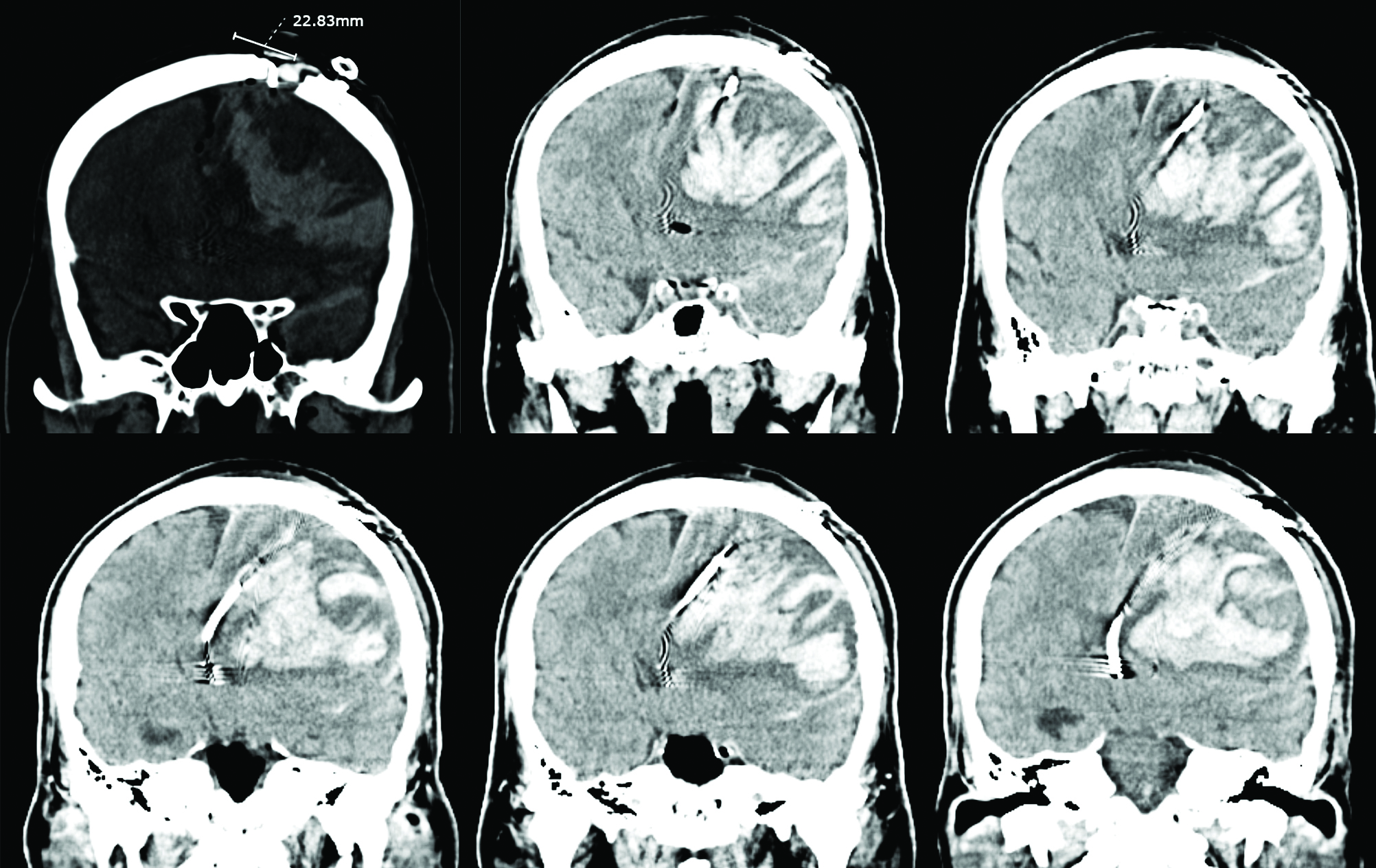

We diagnosed our patient’s hemorrhagic venous infarction on the basis of criteria reported by Morishita et al.,1) which in our case were development of subacute neurologic deficits and CT evidence of significant cerebral edema surrounding the characteristic subcortical hemorrhage.
It is understood that sacrifice of a major cortical vein can result in symptomatic venous infarction.2) In the case we describe, however, no superficial cortical vein was sacrificed for electrode insertion, but we coagulated a venous lacuna within the dura mater during dural opening to achieve hemostasis. Taken together, the intraoperative ICG angiography evidence of delayed venous return, the retrograde venous drainage, and the absence of a coagulated bridging vein beneath the burr hole indicate that this huge postsurgical hemorrhagic infarction was probably due to the occlusion of a venous lacuna. The venous lacunae exist in the dura mater over the convexity 0.5–3.0 cm lateral to the superior sagittal sinus, and receive predominantly the drainage of the meningeal veins, but there have been reports of direct communication between the cortical veins and the lacunae.3–5) In our case, intraoperative ICG angiography revealed drainage directly from a cortical vein into a lateral venous lacuna. Thuy et al.4) reported direct communication between the vein of Trolard and a lateral lacuna of the superior sagittal sinus. Dural veins and superior sagittal sinus were not particularly evaluated in our patient preoperatively, and thus we surmised that the hemorrhagic infarction was due to the occlusion of the lateral lacuna that most likely drained cortical veins from a large part of the left frontal lobe.
Venous infarction is one of the possible causes of intracerebral hemorrhage after DBS surgery, and deficits that are attributable to a venous infarction normally resolve completely or almost completely.2) Permanent serious neurological sequelae are very rare, and we believe that such a catastrophic hemorrhagic venous infarction may also occur with other burr hole surgeries, such as ventriculoperitoneal shunting and endoscopic third ventriculostomy. We wish to alert neurosurgeons to the possibility that interruption of an intradural vein for burr hole surgery can result in a catastrophic hemorrhagic venous infarction, and emphasize that to avoid such disastrous hemorrhagic venous infarction after burr hole surgery, a contrast- enhanced imaging study should be performed preoperatively to carefully evaluate not only the vessels in the brain but also the dural vessels, and a burr hole should be drilled more than 3 cm outward from the midline.
The patient/next of kin/guardian have consented to the submission of the case report for submission to the journal.
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices in the article. All authors who are members of The Japan Neurosurgical Society (JNS) have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.