2021 Volume 8 Issue 1 Pages 493-503
2021 Volume 8 Issue 1 Pages 493-503
Neurenteric cysts (NCs) are rare benign congenital neoplasms in the central nervous system that originate from endodermal elements. NCs are more commonly located in the spine than in the brain. Although almost all intracranial NCs are found in the posterior fossa, some have reported supratentorial NCs. The complete excision of the cyst wall is suggested as a curative treatment; however, endoscopic treatment is less discussed. We present a supratentorial intraparenchymal NC in the frontal lobe treated by neuroendoscopic fenestration and review the literature regarding supratentorial NCs. A 43-year-old woman presenting with right hemiparesis and gait disturbance who was found to have a huge cystic lesion with calcification in her left frontal lobe underwent endoscopic fenestration to the ipsilateral lateral ventricle and biopsy. The histopathological diagnosis was consistent with NC. Postoperatively, her right hemiparesis and gait disturbance disappeared. Postoperative MRI showed shrinkage of the cyst. She was discharged without neurological deficits and no recurrence was seen 1 year after surgery. To the best of our knowledge, there have been no reports of a supratentorial intraparenchymal NC treated by neuroendoscopic fenestration. Minimally invasive treatments, such as neuroendoscopic cyst fenestration, can be considered depending on the location of the cyst.
Neurenteric cysts (NCs) are rare benign congenital neoplasms in the central nerve system that originate from endodermal elements.1) NCs are more commonly located in the spine than in the brain (3:1).2) Almost all intracranial NCs are found in the posterior fossa, though some have reported supratentorial NCs.1) In this report, we present the case of a 43-year-old woman who presented with right hemiparesis and gait disturbance and who was found to have a huge cystic lesion with calcification in her left frontal lobe and underwent endoscopic fenestration to the ipsilateral lateral ventricle and biopsy. The histopathological diagnosis was consistent with NC. We also review the characteristics of supratentorial NC.
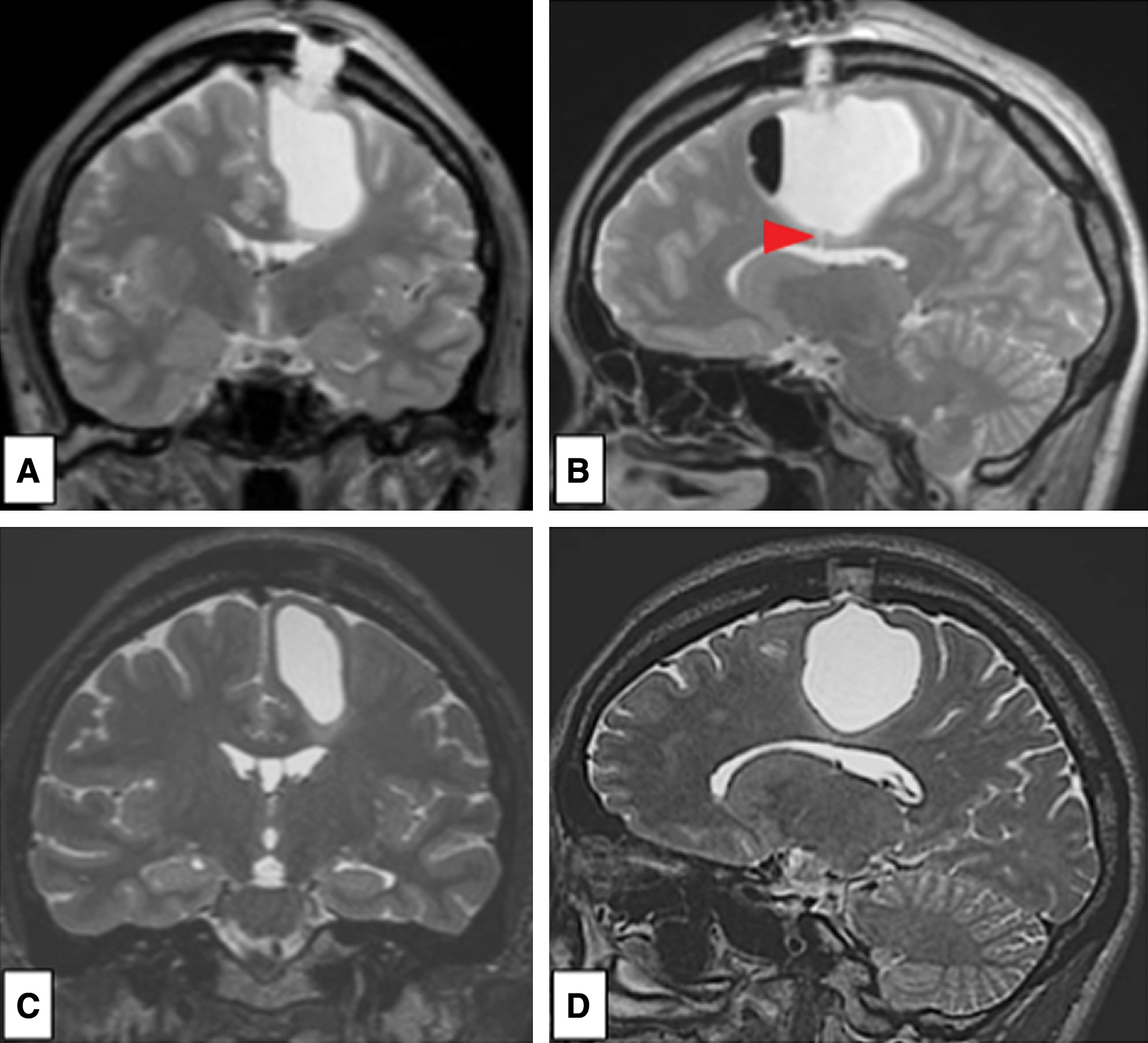
A 43-year-old woman was referred to our hospital with a 9-month history of progressive right hemiparesis and gait disturbance. Computed tomography (CT) demonstrated a huge cyst in the left frontal lobe and several calcified lesions surrounding the cyst wall (Fig. 1A). Magnetic resonance imaging (MRI) revealed an intra-axial cyst measuring 6 × 4 × 6 centimeter in the left frontal lobe. The cyst was adjacent to the left lateral ventricle. The cyst fluid was the same intensity as cerebrospinal fluid. No contrast enhancement nor perilesional edema were shown (Fig. 1B–1G).

We attempted to perform the neuroendoscopic fenestration of the cyst to the ipsilateral lateral ventricle and biopsy of the cyst wall. Under general anesthesia, the burr hole was made using a cranial perforator and extended up to about 20 mm diameter with a Kerrison rongeur. The peel-off sheath (22 Fr; Medikit, Tokyo, Japan) was introduced into the cyst via the left superior frontal gyrus. The fenestration was achieved using a flexible endoscope (Olympus VEF-V; Olympus Medical Systems, Tokyo, Japan) with the guidance of the navigation system (StealthStation; Medtronic, Fridley, MN, USA) because the lateral ventricle could not be seen through the cyst (Fig. 2A). The fenestrated hole was expanded by the balloon (Fig. 2B). The communication from the cyst to the lateral ventricle was achieved (Fig. 2C) and the lateral ventricle bulged to the cyst after fenestration (Fig. 2D). The white calcified nodule was identified and removed with forceps (Fig. 2E and 2F). The T-shaped gelatine sponge (Spongel; LTL Pharma, Tokyo, Japan) was packed into the trajectory, and the wound was closed with a burr hole button.

The histopathological examination showed that the cyst wall was lined by a simple epithelial layer. The epithelial cells were columnar and ciliated (Fig. 2G). On the immunohistochemical staining, the cyst wall cells were positive for cytokeratin (Fig. 2H). The white nodule lesion showed calcification under the epithelium (Fig. 2I). A diagnosis of NC was made. Postoperatively, her right hemiparesis and gait disturbance were dramatically disappeared. She was discharged 7 days after the surgery without neurological deficits. The postoperative MRI showed shrinkage of the cyst (Fig. 3A and 3B). 3-month later MRI showed further shrinkage of the cyst although the fenestration became unclear (Fig. 3C and 3D). No recurrence was seen 1 year after surgery.
NCs, also called endodermal cyst, enterogenous cyst, neuroenteric cyst, and bronchogenic cyst,3) are rare benign endodermal lesions of the central nervous system and account for 0.01% of central nervous system (CNS) tumors.4–6) NCs are more commonly found in the spine than in the brain.7–9) The frequency of intracranial NC is reported between 10% and 17.9% in all NCs 10) and approximately less than one-third in the brain compared with the spine.2) Most intracranial NCs are found in the posterior fossa, though only 14% of intracranial NCs are supratentorially located.11) Gauden et al.11) reported that a median age at presentation of intracranial NC is 34 years, which suggests that intracranial NC is more common in adulthood compared to the intraspinal NC, which commonly occurs during the pediatric years. The size of a supratentorial NC is significantly larger than that of an NC in the posterior fossa.1) The reason the median age of intracranial NC is higher and the size is bigger than that of intraspinal NC may be described by the lower tolerance of local mass effects on the spinal cord.11) An intracranial NC takes a longer time to grow large enough to show neurological symptoms than an intraspinal NC. In the present case, the NC that remained in the patient’s left frontal lobe finally became large enough to cause hemiparesis and gait disturbance. Non-existence of edema supported the fact that the growth speed was slow, although the cyst was huge.
The etiology of the NC is unknown.7,12) Since epidemiology shows that NCs are common in the spine, one of the theories that is generally accepted suggests that they arise at the time of notochordal development during the transitory existence of the neurenteric canal. The notochord and foregut fail to separate during the process of excalation, which causes the endodermal cells remain in the notochord. These cells ultimately become the cyst.7,13–16) However, this theory cannot explain supratentorially located NCs because supratentorial tissues originate from ectoderm. Mittal et al. proposed that anomalous endodermal cell migration occurs dorsally through the primitive neurenteric canal into the ectoderm. Endodermal cells can freely travel in cephalad and in a more lateral localization. This theory also explains the progressively increasing incidence of supratentorial, posterior fossa, and intraspinal NCs.3)
Mittal et al. revealed the lateralization of intracranial NCs, of which 63% of the cases were located in the midline, 29% had laterality, and 8% were undefined.3) To summarize more of the characteristics of supratentorial NCs, we performed a PubMed systematic article search using the key words “neurenteric cyst,” “endodermal cyst,” “neuroenteric cyst,” “supratentorial,” and “intracranial” in different combinations. Reference lists of the selected articles were manually searched to define the characteristics of the cases. In all, 45 studies with 57 patients were finally selected. All the selected patients, along with the one patient reported in the article above, are on the list (Table 1).1,3–5,17–57) Among the 58 cases, the average age was 42.1 (range: 5–78 years). The male to female sex ratio was 1: 0.71. The lateralization is almost equal. The average diameter ± standard deviation (SD) was 5.06 ± 2.29 cm. The incidence in the frontal lobe is 37 cases (64%). We conclude that supratentorial NCs tend to be frontal and not lateral. Some cases of supratentorial NC had two or more neurological symptoms. The most common presenting symptoms were headache (25/57), seizure (20/57), motor deficit (11/57), and visual symptoms (6/57). In all cases of the supratentorial NCs, craniotomies were performed. No cases were treated with neuroendoscopy. Of the reported cases undergone craniotomy, 17 cases (29.8%) had complications after surgery. The most frequent complication was a seizure (11 cases, 19.2%), and two cases of the seizure were required re-intubation. Postoperative neurological deficits were in two cases (3.5%). Postoperative hemorrhage, fluid collection requiring reoperation, infection, and syndrome of inappropriate antidiuretic hormone secretion was in one case (1.7%), respectively. Only two cases of supratentorial NCs mentioned the possibility of malignant transformation. One case was a 45-year-old female with a well-differentiated papillary adenocarcinoma in association with an NC. She was required reoperation 16 months after the first surgery, but the second surgical specimen consisted of the benign cyst wall.5) The other case was a 58-year-old female with an NC exhibiting malignant transformation into an invasive mucinous papillary cystadenocarcinoma and no recurrence was shown 38 months after gross total resection.22) To the best of our knowledge, there were no reports that mentioned the evidence of chemotherapy or radiotherapy.
| N | Author | Year | Age (y.o.) | Sex | Side | Location | Size | Treatment | Recur. | Reop. | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | List et al31) | 1961 | 40 | F | Bil. | Interhemispheric fissure | N/A | Surgery (C) | + | + | Improved |
| 2 | Vuia51) | 1976 | 27 | M | Lt. | Frontal | 100 mL | Surgery (C) | N/A | N/A | Improved |
| 3 | Walls et al52) | 1986 | 40 | F | Both | Multiple lesions | N/A | Surgery (C) | N/A | N/A | Improved |
| 4 | Campbell et al17) | 1991 | 33 | F | Rt. | Frontoparietal | N/A | Surgery (C) | N/A | N/A | Improved |
| 5 | Dauch et al21) | 1991 | 35 | M | Rt. | Frontal | 170 mL | Surgery (C) | N/A | N/A | Improved |
| 6 | Scaravilli et al46) | 1992 | 36 | M | Lt. | Optic nerve | 2 cm | Surgery (C) | N/A | N/A | Unchanged |
| 7 | Leventer et al30) | 1994 | 23 | F | Lt. | Superior orbital fissure | N/A | Surgery (C) | + | + | Improved |
| 8 | Bavetta et al56) | 1996 | 28 | M | Rt. | Frontal | 5 cm | Surgery (C) | N/A | N/A | Improved |
| 9 | Büttner et al57) | 1997 | 28 | M | Midline | Third ventricle | 1.2 cm | Surgery (C) | N/A | + | Improved |
| 10 | Ho et al5) | 1998 | 45 | F | Rt. | Parietal | 8 cm | Surgery (C), fenestration | N/A | + | Partial improved |
| 11 | Sampath et al45) | 1999 | 27 | M | Lt. | Parasellar | N/A | Surgery (C) | N/A | N/A | Improved |
| 12 | Mishra et al34) | 2000 | 19 | F | Midline | Septum pellucidum | N/A | Surgery (C) | N/A | + | Improved |
| 13 | Morgan et al36) | 2001 | 30 | F | Rt. | Oculomotor nerve | 1.3 cm | Surgery (C) | N/A | + | Improved |
| 14 | Cheng et al4) | 2002 | 49 | M | Rt. | Frontal | N/A | Surgery (C) | N/A | N/A | Improved |
| 15 | Christov et al20) | 2004 | 31 | F | Rt. | Frontoparietal | N/A | Surgery (C) | N/A | + | Improved |
| 16 | Kachur et al27) | 2004 | 35 | F | Rt. | Frontal | 4 cm | Surgery (C) | N/A | N/A | Improved |
| 17 | Tan et al50) | 2004 | 68 | F | Lt. | Frontal | 6.5 cm | Surgery (C) | N/A | + | Improved |
| 18 | Preece et al1) | 2006 | 72 | F | Lt. | Frontal | 8.8 cm | Surgery (C) | N/A | N/A | N/A |
| 19 | 34 | M | Lt. | Frontal | 9 cm | Surgery (C) | N/A | N/A | N/A | ||
| 20 | 78 | F | Rt. | Frontal | 7 cm | Surgery (C) | N/A | N/A | N/A | ||
| 21 | 48 | M | Lt. | Frontal | 8 cm | Surgery (C) | N/A | N/A | N/A | ||
| 22 | 78 | M | N/A | Frontal | 7 cm | Surgery (C) | N/A | N/A | N/A | ||
| 23 | Neckrysh et al39) | 2006 | 70 | M | Midline | Sellar | 5 cm | Surgery (C) | N/A | N/A | Improved |
| 24 | Stubenvoll et al48) | 2006 | 25 | M | Rt. | Frontal | 5.5 cm | Surgery (C), fenestration | N/A | N/A | Improved |
| 25 | Miyagi et al35) | 2007 | 63 | M | Rt. | Parietal | 5 cm | Surgery (C) | N/A | N/A | Improved |
| 26 | Takumi et al49) | 2008 | 32 | M | Lt. | Frontal | 2 cm | Surgery (C) | N/A | N/A | Improved |
| 27 | Marchionni et al33) | 2008 | 20 | F | Lt. | Multiple | N/A | Surgery (C) | N/A | N/A | Improved |
| 28 | Dunham et al22) | 2009 | 58 | F | Rt. | Parietal | 4.5 cm | Surgery (C) | N/A | N/A | Malignant transformation |
| 29 | Basheer et al55) | 2010 | 54 | M | Rt. | Parietooccipital | N/A | Surgery (C) | N/A | N/A | Improved |
| 30 | Krishnamurthy et al29) | 2010 | 32 | M | Rt. | Frontoparietal | N/A | Surgery (C) | N/A | N/A | Improved |
| 31 | 44 | F | Lt. | Frontoparietal | N/A | Surgery (C) | N/A | N/A | Improved | ||
| 32 | Mittal et al3) | 2010 | 76 | F | Rt. | Frontoparietal | 4 cm | Surgery (C) | N/A | N/A | Improved |
| 33 | Reddy et al43) | 2010 | 20 | M | Lt. | Temporal-posterior fossa | N/A | Surgery, craniectomy | N/A | N/A | Improved |
| 34 | Jhawar et al25) | 2011 | 41 | M | Rt. | Temporal | N/A | Surgery (C) | N/A | N/A | Improved |
| 35 | Little et al32) | 2011 | 70 | M | Midline | Sellar | 4 cm | Surgery (C) | N/A | N/A | Improved |
| 36 | Natrella et al38) | 2012 | 45 | M | Lt. | Frontal | 3.7 cm | Surgery (C) | + | N/A | Improved |
| 37 | Arabi et al53) | 2013 | 67 | M | Lt. | Frontal | 4.8 cm | Surgery (C) | N/A | N/A | Improved |
| 38 | Janczar et al24) | 2014 | 33 | F | Lt. | Frontal | 3.5 cm | Surgery (C) | N/A | N/A | Improved |
| 39 | Junaid et al26) | 2014 | 35 | M | Rt. | Frontotemporoparietal | 6.7 cm | Surgery (C) | N/A | N/A | Improved |
| 40 | Kitamura et al28) | 2014 | 28 | M | Lt. | Frontal | N/A | Surgery (C) | N/A | + | Improved |
| 41 | Salvetti et al44) | 2014 | 28 | F | Midline | Third ventricle | 1.1 cm | Surgery (C) | N/A | N/A | Improved |
| 42 | Chakraborty et al18) | 2016 | 71 | M | Lt. | Frontal | 6.4 cm | Surgery (C) | N/A | N/A | Improved |
| 43 | 68 | F | Lt. | Frontal | 5.5 cm | Surgery (C) | N/A | N/A | Improved | ||
| 44 | 39 | F | Lt. | Frontal | 2.3 cm | Surgery (C) | N/A | N/A | Unchanged | ||
| 45 | Rangarajan et al42) | 2016 | 52 | F | Lt. | Frontal | N/A | Surgery (C) | N/A | N/A | Improved |
| 46 | 32 | M | Midline | Frontal | N/A | Surgery (C) | N/A | N/A | Improved | ||
| 47 | Chen et al19) | 2016 | 63 | M | Rt. | Frontal | 11.9 mL | Surgery (C) | N/A | N/A | N/A |
| 48 | 6 | M | Lt. | Parietotemporal | 140 mL | Surgery (C), fenestration | + | N/A | N/A | ||
| 49 | 65 | F | Rt. | Parietal periventricle | 25.4 mL | Surgery (C) | N/A | N/A | N/A | ||
| 50 | 0 | M | Rt. | Frontotemporal base | 141.8 mL | Surgery (C), fenestration | + | N/A | N/A | ||
| 51 | Bao et al54) | 2016 | 43 | M | Lt. | Temporal | 10 mL | Surgery (C) | N/A | N/A | Improved |
| 52 | Góes et al23) | 2018 | 46 | M | Lt. | Frontoparietal | 9 cm | Surgery (C), fenestration | N/A | N/A | Improved |
| 53 | 49 | M | Lt. | Frontal | 5 cm | Surgery (C) | + | N/A | Improved | ||
| 54 | Dias et al40) | 2019 | 14 | M | Rt. | Frontal | 136 mL | Surgery (C) | N/A | N/A | Improved |
| 55 | Agrawal et al47) | 2019 | 30 | F | Rt. | Frontal | N/A | Surgery (C), fenestration | N/A | N/A | Improved |
| 56 | Nagata et al37) | 2019 | 48 | M | Lt. | Frontotemporal | N/A | Surgery (C) | N/A | N/A | Improved |
| 57 | Oliveira et al41) | 2019 | 30 | M | Rt. | Frontal | N/A | Surgery (C) | N/A | N/A | N/A |
| 58 | Present case | 2020 | 43 | F | Lt. | Frontal | 6 cm | Endoscopic fenestration (B) | – | – | Improved |
B: burr hole surgery, Bil.: bilateral, C: craniotomy, F: female, Lt.: left, M: male, Rt.: right, Recur.: recurrence, Reop.: reoperation, N/A: not available, y.o.: year old.
NCs showed sharply demarcated cystic lesions on CT.1) The content of the cyst usually has a lower density on CT imaging and is more hyperintense on T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) imaging.2,58) A cyst which has protein-rich content shows high density on CT and is hypertense on MRI T1-weighed images.58) Because intraparenchymal cysts are relatively rare and difficult to diagnose on CT or MRI, histopathological diagnosis is important.
The differential diagnosis for intracranial NC includes epidermoid cyst, dermoid cyst, arachnoid cyst, neuroglial cyst, and other endodermal cysts such as Rathke cyst and colloid cyst.2,15,59,60) It is difficult to differentiate them by imaging only because they usually show similar characteristics on CT and MRI.3)
NC is a cyst lined by simple columnar epithelium, which may be ciliated. The epithelium may be converted into a low cuboidal state by chronic pressure although originally columnar.61) The epithelium types are divided into three; gastrointestinal-type epithelium in approximately 50%; ciliated respiratory epithelium in 17%; and mixed types in about 33%.1,62) In the immunohistochemistry, the epithelium is positive for cytokeratin and epithelial membrane antigen, which are epithelial markers, and carcinoembryonic antigen, which is a marker of the embryonic gastrointestinal tract.50) It is negative for neuron-specific enolase, synaptophysin, glial fibrillary acidic protein, and S-100, which are markers of nervous system or ectoderm tissue.27) In this case, the epithelium was single-layered ciliated cells and immunopositive for cytokeratin and EMA, though it was immunonegative for S-100. All the macroscopic characteristics agreed with the reported ones and revealed that the cyst originated from endoderm.
Tan et al.50) reported the first NC with calcification, and Preece et al. followed.1) However, the previous reports did not histopathologically confirm calcification. This case is the first case reporting the histopathology with calcification and the cyst wall together. It is surprising that the normal nerve tissue was present between the cyst wall and the calcification, which suggests that calcification may have originated from brain tissue by longstanding compaction.
NCs are rarely symptomatic and consequently can be followed with serial imaging.58) When symptomatic, treatment choices would include decompression by gross total resection of the cyst wall, partial resection, fenestration, or aspiration. To prevent recurrence and eliminate the possibility of malignant transformation, complete excision of the cyst wall is emphasized.63) However, strong cyst adhesions to the surrounding neurovascular structures may cause limitations for complete excision,23) so choices of treatment should be considered, respectively. Chen et al. analyzed 12 NC cases retrospectively and concluded that risk factors of recurrence include patients with an age of 30 years or less, subtotal resection, and larger size of supratentorial NC than 30 mL.19)
Because the origin of the cyst could be intraparenchymal, and not the ventricle system, we speculated that the lateral ventricle could not be seen through the cyst in spite of the fact that the cyst was close to it. Therefore, we prepared StealthStation with EM Flexible stylet for confirmation of the adequate site to puncture from the cyst to the lateral ventricle. However, the cyst fenestration to the ventricle or the cistern should be abandoned when the cyst is not close to the ventricle system or the cistern to prevent unexpected complications. Although endoscopic fenestration of the NC has not been reported before, we believe that the cyst fenestration and biopsy by endoscopy is less invasive treatments and one of the ways to histopathologically diagnose. Occasionally, craniotomy and cyst wall removal may have a risk of subdural hematoma due to brain sagging followed by the injury of the bridging veins or brain injury if strong adhesion exists between the cyst wall and the brain parenchyma. On the other hand, the rate of the recurrence should be higher in the treatment of the fenestration compared to the craniotomy and cyst wall removal because the cyst wall continues to produce fluid, and the occlusion of the fenestration can occur in a long-time follow-up. Additional treatments, such as craniotomy and cyst removal, Ommaya reservoir placement, shunt placement, endoscopic fenestration, and stent placement between cyst and ventricle, should be discussed at the time of the recurrence.
We reported a rare case of supratentorial intraparenchymal NC in the left frontal lobe. The histopathological diagnosis and cyst fenestration were achieved by a neuroendoscopic procedure. Our patient has shown no evidence of recurrence by a year follow-up after surgery, but long-term follow-up is necessary because of the possibility of recurrence.
None of authors have any disclosure to report.