2021 Volume 8 Issue 1 Pages 631-635
2021 Volume 8 Issue 1 Pages 631-635
Nonocclusive mesenteric ischemia (NOMI) is a rare but life-threatening post-stroke complication. This is the first case series report of NOMI after stroke, describing its characteristics and the difficulty of diagnosis. We retrospectively reviewed 367 stroke patients from April 2018 to May 2019 in our hospital. We identified six patients (1.6%) with NOMI after stroke and described their clinical presentation, including characteristics, vital signs, laboratory parameters, treatment, and outcomes. The mean interval between stroke onset and diagnosis of NOMI was 4.6 days (range, 3–10 days). Five patients had disturbance of consciousness caused by stroke, and therefore the major complaints and symptoms associated with NOMI were nonspecific, possibly resulting in delayed diagnosis and treatment. All patients had a high respiratory rate (≥22 breaths/min). C-reactive protein and lactate levels were high (mean, 21.6 mg/L and 2.4 mmol/L, respectively). All patients underwent emergent abdominal operations. Four patients were discharged with modified Rankin Scale scores ≥4, and two patients died. NOMI can be a fatal post-stroke complication and is challenging to diagnose. Further investigations should be conducted to determine the most efficient way to diagnose NOMI after stroke.
Nonocclusive mesenteric ischemia (NOMI) is a mesenteric ischemia or necrosis without thrombosis in the mesenteric arteries. The majority of cases involve spasm of branches of the superior mesenteric artery (SMA) that supply the small intestine and proximal colon. NOMI accounts for 5–15% of cases of acute mesenteric ischemia.1) Its incidence has increased due to mesenteric arterial vasoconstriction secondary to hypotension in cases of shock, septicemia, dehydration, heart surgery, and major abdominal surgery.2) The disease has a high mortality rate of 63–81%.3,4) Because of the uncertain clinical status and the fact that many NOMI patients are misdiagnosed, the prognosis of NOMI is extremely poor and its mortality rate has not changed over time.5,6) Clinically, NOMI can present with abdominal pain, nausea, vomiting, gastrointestinal hemorrhage, and ileus symptoms, but the characteristic early symptoms and laboratory test results are unclear.7–9) Although the number of stroke patients with risk factors for NOMI is increasing, descriptions of NOMI as a complication of stroke are poor. The diagnosis is often delayed because some stroke patients cannot describe their symptoms because of unconsciousness.
We report a case series of six patients hospitalized at our institution from April 2018 to May 2019 for NOMI after stroke.
We surveyed 367 patients with stroke treated in our hospital between April 2018 and May 2019. We extracted data from patients who developed NOMI and recorded their age, gender, vital signs, blood tests, treatment, and outcome. We mainly referred to contrast-enhanced abdominal CT scan to diagnose NOMI. To evaluate vital signs, we referred to quick Sequential Organ Failure Assessment (qSOFA), a bedside prompt to identify patients with suspected infection who are at higher risk for a poor outcome outside the intensive care unit. The qSOFA uses three criteria, assigning one point each for low blood pressure (systolic blood pressure [SBP] ≤100 mmHg), high respiratory rate (≥22 breaths/min), and altered mentation (Glasgow Coma Scale [GCS] <15). To evaluate outcome, we used the discharge modified Rankin Scale (mRS).
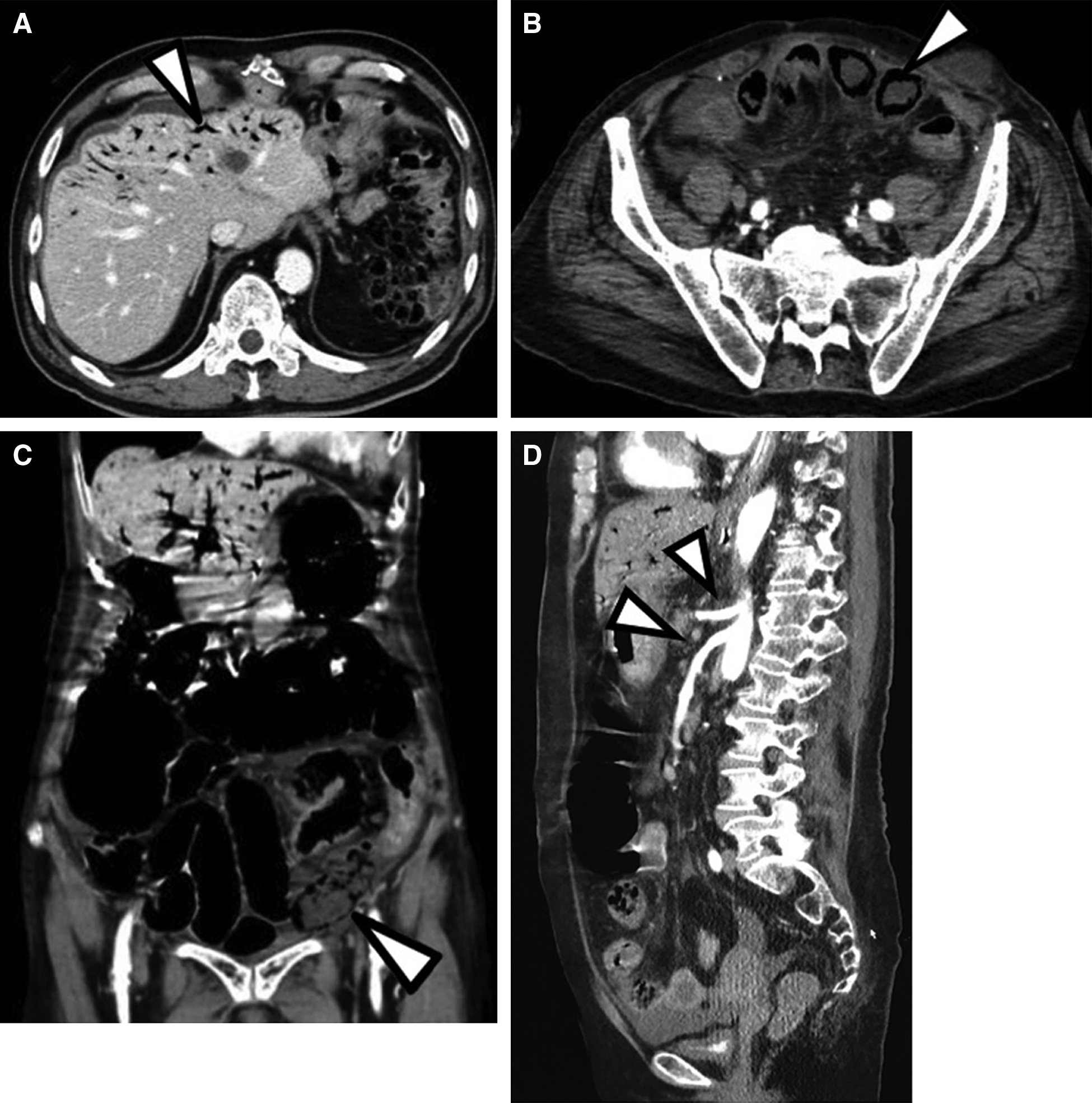
Six patients (1.6% of total patients; mean age, 75 years; range, 61–82 years; 50% males, 50% females) were enrolled in the study. Two patients suffered from subarachnoid hemorrhage (World Federation of Neurosurgical Societies grade 4), two patients suffered from cerebral infarction (CI) after endovascular therapy (EVT), and two patients each suffered from intracerebral hemorrhage (ICH) and CI under observation. The mean interval from stroke onset to development of NOMI was 4.6 days (range, 3–10 days). One patient had low blood pressure (SBP ≤100 mmHg). Five patients had altered mentation (GCS <15), and one patient complain of abdominal pain. All patients had abdominal distension and tenderness. All patients had a high respiratory rate (≥22 breaths/min). C-reactive protein (CRP) and lactate levels were high (mean, 21.6 mg/L and 2.4 mmol/L, respectively). All patients were diagnosed by the presence of ischemic bowel signs (portal venous gas, pneumatosis intestinalis, and absence of bowel wall enhancement) without occlusion or thrombus of the mesenteric arteries in the CT scan findings (Fig. 1). All patients underwent emergency abdominal operations. Five patients underwent intestinal resection; one patient could not undergo intestinal resection because the extent of necrosis was too great. Pathological findings in the resected intestines showed nonocclusive and segmental enterointestinal damage with ulcer and necrosis on the membrane (Fig. 2). These were characteristic pathological findings. Four patients were discharged with mRS scores ≥4, and two patients died during the postoperative period in the hospital. The results are summarized in Table 1.

| Feature | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Age (years) | 75 | 61 | 78 | 74 | 82 | 79 |
| Gender | Female | Female | Male | Male | Male | Female |
| Risk factor of NOMI | Renal insufficiency | None | Diabetes, major abdominal surgery | Low cardiac output, major abdominal surgery | None | Renal insufficiency, low cardiac output |
| Stroke | SAH (WFNS grade 4) | SAH (WFNS grade 4) | CI (M1 occlusion) | CI (ICA occlusion) | ICH (putaminal hemorrhage) | CI (M2 occlusion) |
| Stroke treatment | Clipping (aneurysm at M1/M2 bifurcation) | Clipping (aneurysm at M1/M2 bifurcation) | EVT | EVT | Observation | Observation |
| Abdominal symptoms | Abdominal tenderness, distension | Abdominal tenderness, distension | Abdominal pain, tenderness, distension | Abdominal tenderness, distension | Abdominal tenderness, distension | Abdominal tenderness, distension |
| NOMI treatment | Bowel resection | Bowel resection | Bowel resection | Bowel resection | Exploratory laparotomy | Bowel resection |
| Period (days) from stroke onset to NOMI | 3 | 10 | 3 | 4 | 5 | 3 |
| mRS score at discharge | 6 | 4 | 4 | 5 | 6 | 4 |
CI: cerebral infarction, EVT: endovascular therapy, ICH: intracerebral hemorrhage, mRS: modified Rankin scale, M1: sphenoidal or horizontal segment of the middle cerebral artery, M2: insular segment of the middle cerebral artery, NOMI: nonocclusive mesenteric ischemia, SAH: subarachnoid hemorrhage, WFNS: World Federation of Neurosurgical Societies.
NOMI was first described by Ende in 1958 as a type of mesenteric ischemia with no evident occlusion of the mesenteric arteries.10) It is a rare, devastating disease, with reported risk factors including older age, low cardiac output, diabetes, sepsis, use of vasoconstrictors, use of diuretics, use of digitalis, cardiac or major abdominal surgery, renal insufficiency, and conditions that cause vasoconstriction (e.g., shock, dehydration, hypotension, and hemodialysis).2,11)
NOMI is a difficult disease to diagnose because the symptoms are so nonspecific that treatment is often delayed. Acute abdominal pain, nausea, and vomiting may be the only early presenting symptoms. Leukocytosis and elevated lactate levels are often observed, but these are also nonspecific, and elevated lactate levels may be a delayed sign of progressive disease. At present, there is no early biological marker that is commonly used in clinical practice for diagnosis of NOMI.12) Changes in mental status are reported to occur in approximately one-third of elderly patients with acute mesenteric ischemia.13) Patients are often in an altered state of consciousness after stroke, so it is difficult to diagnose NOMI based on the state of consciousness. NOMI has the poorest survival among the types of mesenteric ischemia, with a high mortality rate.
Stahl et al. reported that oxygenation index, norepinephrine requirement, and SOFA score changed in the process of establishing the diagnosis and that other parameters, mainly reflecting severe tissue injury such as creatinine kinase and transaminases, changed rather late during the disease course.4)
When NOMI is suspected clinically and there is no perforation or necrosis of the bowel wall, immediate selective angiography of the mesenteric arteries is important. If NOMI is confirmed, transcatheter infusion of vasodilators may be most useful. Vasodilators are administered through an arteriographic catheter, typically placed into the SMA. Papaverine and prostaglandin E1 are the most commonly used agents, but their availability is limited.14) Winzer et al. reported that local intra-arterial infusion of papaverine in patients with NOMI significantly increased survival in comparison with conservative treatment. However, this study also reported that high lactate levels, low pH and high base excess, and high demand for catecholamines were associated with poor outcome.15)
In this study, patients are complicated by NOMI after subarachnoid hemorrhage and CI with indication for surgery, and after cerebral hemorrhage and CI with indication for conservative treatment. Some patients were elderly and had diabetes as risk factors, but others did not have the above risk factors and developed NOMI. Therefore, there is a possibility that stroke itself can cause vasoconstriction. This is the first report of NOMI as a complication of various types of stroke. The common findings of NOMI were high CRP and lactate levels, altered mentation, and high respiratory rate. In particular, all six patients had a high respiratory rate. Hyperventilation is an emergency response of the lung to metabolic acidosis. Taking deeper, longer breaths enables the lungs to expel more acidic carbon dioxide. Of course, patients with severe heart failure and pneumonia also have a high respiratory rate. However, these patients also have hypoxemia and require oxygen. Therefore a high respiratory rate may be important for early diagnosis of NOMI. All cases were diagnosed by the presence of ischemic bowel signs without occlusion or thrombus of the mesenteric arteries in CT scan findings, and all six patients with NOMI underwent emergency laparotomies. After the bowel resection, delayed oral intake leads to malnutrition and delayed rehabilitation leads to a progressive decline in ADL. These make mRS score at discharge worse. Early diagnosis is of utmost importance to avoid intestinal ischemia and subsequent multiorgan failure or potentially lethal complications such as bowel necrosis and perforation.11,12) Further investigations should be conducted to determine the most efficient way to diagnose NOMI after stroke before the patient develops intestinal necrosis with metabolic acidosis.
Regardless of the way we treat stroke, we need to recognize that NOMI is one of the life-threatening complications after various types of stroke. In comparison with other diseases, it is difficult to diagnose NOMI based on consciousness in stroke patients. We should perform a careful physical examination and measurement of laboratory parameters, particularly respiratory rate, lactate and CRP levels, and altered mentation, to diagnose NOMI after stroke.
All procedures in this study were in accordance with the ethical standards of the institutional research committee “Tokyo Medical University Hachioji Medical Center,” approval number: T2020-0175; approval date: October 2, 2020.
Informed consent was obtained from the patients included in the study.
We would like to give a special thanks to Enago (www.enago.jp) for the English language review.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.