2021 Volume 8 Issue 1 Pages 697-703
2021 Volume 8 Issue 1 Pages 697-703
Solitary fibrous tumor (SFT) or hemangiopericytoma (HPC) is a rare fibroblastic tumor of mesenchymal origin. SFT or HPC comprises <1% of all primary central nervous system tumors. SFT or HPC of the sellar or suprasellar region is even more unusual. We herein report a sellar SFT or HPC in an octogenarian who achieved favorable progress with partial removal followed by fractionated gamma knife radiosurgery. An 87-year-old woman presented with occasional headache and visual field defects. A rapidly growing tumor of the sella turcica was diagnosed. The patient underwent endoscopic transnasal transsphenoidal surgery; however, only partial resection of the tumor was possible, as it was fibrous and hard with increased vascularity. A histological examination confirmed the tumor to be grade II SFT or HPC. Two months after the resection, the residual tumor grew rapidly. Given the patient’s advanced age, re-surgery was not the preferred option; thus, fractionated gamma knife radiosurgery (marginal dose, 30 Gy in five fractions) was performed. MRI and visual field examination performed 3 months after irradiation revealed tumor shrinkage and improvement in the visual field, respectively. One year and three months after irradiation, the tumor continued to shrink and her visual field had improved. Taking age into consideration, partial resection with fractionated gamma knife radiosurgery was the more appropriate choice for both local tumor control and the safety of the optic apparatus.
Solitary fibrous tumors (SFTs) or hemangiopericytomas (HPCs) comprise <1% of all primary central nervous system (CNS) tumors.1) They are mostly dural-based tumors that are thought to arise from meningeal capillary pericytes and are therefore found in similar locations to meningiomas.2) However, the sellar and suprasellar SFT or HPC are even more unusual. To date, there are only 17 case reports of sellar and suprasellar SFT or HPC published in the English literature since 2001.3–18) Most of these tumors reportedly occur in middle aged patients, with higher preponderance in male individuals, as shown in Table 1. We present the case of a sellar SFT or HPC in an 87-year-old woman who achieved treatment efficacy with fractionated gamma knife radiosurgery after partial resection of the tumor.
| SN | Author | Year | Sex | Age (years) | Variant | Signs/symptoms | Treatment | Follow-up duration/recurrence |
|---|---|---|---|---|---|---|---|---|
| 1 | Cassarino et al. | 2003 | F | 54 | SFT | Headache, VD | Partial resection | NA |
| 2 | Pakasa et al. | 2005 | F | 66 | SFT | VD | Partial resection | Recurrence in 2 years |
| 3 | Kim et al. | 2005 | M | 56 | SFT | VD | Complete resection | NA |
| 4 | Macfarlane et al. | 2005 | M | 33 | SFT | Headache, VD | Partial resection + radiotherapy + chemotherapy | NR up to 18 months |
| 5 | Juco et al. | 2007 | F | 18 | HPC | VD | Partial resection + radiotherapy | NR up to 27 months |
| 6 | Jalali et al. | 2008 | M | 35 | HPC | VD | Complete resection | Recurrence in 6 months |
| 7 | Furlanetto et al. | 2009 | M | 28 | SFT | VD | Complete resection | NR up to 10 months |
| 8 | Das et al. | 2010 | M | 47 | HPC | Headache, VD | Partial resection + radiotherapy | NR up to 3 years |
| 9 | Yin et al. | 2010 | M | 32 | SFT | Headache, VD | Partial resection + radiosurgery | NR up to 44 months |
| 10 | Jain et al. | 2012 | M | 63 | SFT | Headache, VD | Partial resection + radiotherapy | NR up to 8 months |
| 11 | Wu et al. | 2012 | M | 53 | SFT | VD | Partial resection | NR up to 7 months |
| 12 | Esquenazi et al. | 2014 | M | 51 | HPC | VD | Partial resection + repeated surgery | NA |
| 13 | Yang et al. | 2015 | F | 20 | SFT | Headache, VD | Partial resection | NR up to 4 years |
| 14 | Yang et al. | 2015 | M | 22 | SFT | VD | Complete resection | NR up to 2 years |
| 15 | Sahai et al. | 2016 | M | 70 | SFT | VD | Partial resection + radiotherapy | NR up to 1 year |
| 16 | Gibson et al. | 2017 | M | 34 | HPC III | VD | Partial resection + radiosurgery | Recurrence at 2 to 4 months. Died of pulmonary embolism at 7 months |
| 17 | Nesaratnam et al. | 2017 | F | 73 | SFT or HPC III | Headache, VD | Surgery + radiotherapy | NA |
| 18 | Present case | 2020 | F | 87 | SFT or HPC II | Headache, VD |
Partial resection + radiosurgery | NR up to 15 months |
F: female, HPC: hemangiopericytoma, M: male, NA: not available, NR: no recurrence, SFT: solitary fibrous tumor, SN: serial number, VD: visual disturbance.
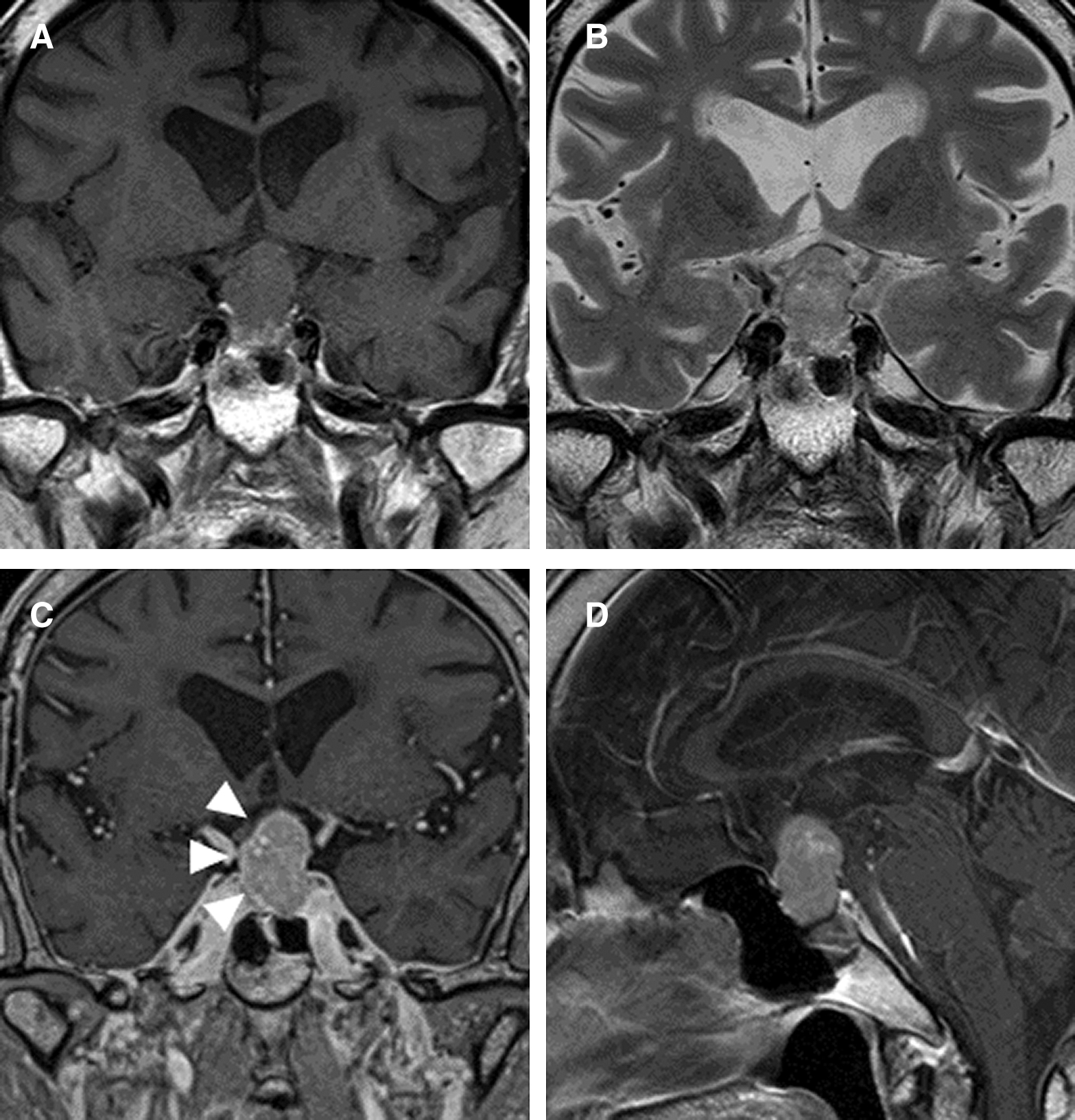
An 87-year-old woman was referred to our neurosurgery department after the identification of a mass in the pituitary fossa. She presented with a history of occasional headaches for several years. She experienced no subjective symptoms, but the Humphrey visual field test indicated visual field defects that were more severe on the left side. Head CT revealed a slightly hyperdense pituitary mass with no calcification and no enlargement of the sella. On MRI, a solid tumor of size 16 mm × 16 mm × 25 mm extending into the sellar and suprasellar regions showed an isointense signal intensity on T1-weighted imaging and iso-to-hyperintense signal intensity on T2-weighted imaging (Fig. 1A and 1B). The left optic nerve was displaced upward by the tumor. The mass displayed heterogeneous enhancement on contrast-enhanced MRI, and the anterior pituitary gland was displaced to the upper right side (Fig. 1C and 1D). No dural tail sign was detected. Unlike what is observed with pituitary adenomas, this tumor showed enhancement from an early stage in the dynamic MRI study. An MRI performed 1.5 years previously due to a headache had revealed a small mass (3 mm × 4 mm × 3 mm) on the left side of the sella, suggesting rapid tumor growth. The patient’s hormonal analysis revealed low cortisol (2.8 mg/dL) and free T4 levels (0.52 ng/dL), for which she was treated with hydrocortisone 5 mg and thyroxine 25 µg, respectively. Her prolactin level was slightly elevated (70.2 ng/mL). The general health condition of the patient rendered her eligible for surgery; hence, we elected to perform surgery first to decompress the optic nerve and to obtain a sample for histopathology.
The patient underwent endoscopic transnasal transsphenoidal surgery. The sellar floor was thick. Upon opening the dura, we observed that the pituitary gland was degenerated. The tumor was encapsulated behind the pituitary gland. A markedly vascular, hard, brownish-gray tumor was observed upon opening the capsule. Given that the tumor could not be removed with suction, we shattered the tumor with a curette. Frozen section biopsy diagnosed a spindle cell tumor, suspected to be SFT or HPC. Considering her advanced age and difficulty in tumor removal, we chose to end the operation with partial resection. A postoperative CT scan showed a residual mass (16 mm × 16 mm × 23 mm) with an extraction cavity (7 mm × 6 mm ×16 mm). A histopathological examination with immunohistochemistry confirmed the lesion to be SFT or HPC, grade II. A staghorn-like vascular network was observed, along with tumor cells with short spindle-shaped or oval nuclei growing irregularly around the blood vessels (Fig. 2A). Nuclear atypia was inconspicuous with absent mitotic figures and necrosis. On immunohistochemistry, the tumor cells were positive for CD34 (Fig. 2B) and STAT6 (Fig. 2C), which was consistent with the diagnosis of SFT or HPC. TTF-1 was negative, which ruled out the possibility of a pituicytoma (Fig. 2D). Her recovery was satisfactory with no postoperative complications. After the operation, her prolactin and cortisol levels were normalized; however, the visual field defects did not improve. After 2 months, the residual tumor showed rapid growth on cranial imaging (Fig. 3A). Given the patient’s advanced age, re-surgery was not the preferred choice; hence, we decided to perform a fractionated gamma knife radiosurgery (marginal dose, 30 Gy in five fractions) using a Leksell Gamma Knife Icon (Elekta Instrument AB, Stockholm, Sweden). The cumulative marginal dose to the optic nerve was planned to be less than 25 Gy. MRI and visual field examination performed 3 months after the irradiation revealed tumor shrinkage (Fig. 3B) and improvement in the visual field, respectively. One year and three months after the irradiation, the tumor continued to shrink (Fig. 3C), and the visual field examination showed further improvement. The patient continued to take levothyroxine, but her other pituitary functions were normal.


Written informed consent was obtained from this patient to publish her case.
Although the origin of SFT or HPC remains unclear, there is a consensus regarding its mesenchymal origin with HPC-like features: monotonous cell population, varying cellularity, and presence of branching blood vessels.19) Ultrastructurally, both SFT and HPC display varying degrees of pericytic, fibroblastic, or myofibroblastic differentiation. Gengler and Guillou clarified that except for myopericytoma, infantile myopericytosis, and HPC of the sinonasal tract, all HPCs, in the absence of pericytic differentiation, are variants of SFT and are analogous to a cellular form of SFT with a morphological continuum.19) These two tumors were considered as a single entity in the 2016 World Health Organization (WHO) classification of tumors of the CNS, as both share inversions at 12q13, fusing the NAB2–STAT6 genes and resulting in nuclear expression of the STAT6 gene.20) Less cellular and highly collagenous tumors are considered grade I (previously known as SFT), and more cellular and less collagenous tumors with characteristic staghorn vasculature are considered grade II (previously known as HPC). Grade III HPC or SFT shows five or more mitoses per 10 high-power fields, which is inclusive of both anaplastic HPC and malignant SFT types.1)
Visual disturbance followed by headache is the most common presenting symptom of sellar SFT or HPC.3–18) As with other suprasellar tumors, these tumors often compress the pituitary gland and stalk. Therefore, pituitary dysfunction is also a common symptom.10,14–16,18) In our case, the anterior pituitary gland was displaced to the upper right side and the patient had hypothyroidism, hypocortisolism, and hyperprolactinemia, which may have been caused by stalk effect at presentation.
Sellar SFTs or HPCs are often mistaken for pituitary adenomas and meningiomas.4,8–15) In our case, early enhancement of the mass lesion on dynamic MRI and rapid tumor growth, indicated by no enlargement of the sella on CT, as well as previous MRI findings, were counter suggestive of the possibility of the lesion being a pituitary adenoma. Similarly, the absence of dural tail sign and calcification excluded the possibility of meningiomas. However, because of the rarity of the tumor and no reliable radiological signs, it was difficult to diagnose SFT or HPC before surgery.
Gross total resection is the gold standard for the treatment of SFT or HPC.21–23) However, partial resection is the most common surgical management, as these tumors are fibrous, hypervascular, and invasive in most cases.3,6,10,11,13,15,16) Although total removal has been achieved in some cases, new visual impairment and pituitary dysfunction have been reported in some of those cases.9,15)
Local recurrences and metastasis are the hallmarks of SFT or HPC, specifically among patients with residual tumors.21–23) However, adjuvant radiation has shown promise for preventing tumor progression and prolonging the time to recurrence.22,23) In previous reports of sellar SFT or HPC, some cases with partial resections received radiotherapy, at a dose of 45-60 Gy, and archived a good clinical outcome.6,7,10,12,16,18)
In sellar SFT or HPC, stereotactic radiosurgery after surgical decompression of the tumor is also effective in preventing tumor progression.11) However, one of the perennial complications of stereotactic radiosurgery for tumors in contact with the optic pathway, such as our case, is radiation-induced optic neuropathy.24) Recently, the use of fractionated gamma knife radiosurgery for perioptic tumors has been accepted. Jee et al. reported favorable visual outcomes and effective tumor control for patients who underwent fractionated gamma knife radiosurgery for perioptic benign tumors.25) The maximum point doses of the optic apparatus, resulting in <1% radiation-induced optic nerve/chiasm neuropathy risks, have been shown to be 20 Gy in three fractions and 25 Gy in five fractions.26) To the best of our knowledge, this is the first case report of fractionated gamma knife radiosurgery for sellar SFT or HPC. Although long-term follow-up is needed, our case provides further evidence for the effectiveness of fractionated gamma knife radiosurgery for both local tumor control of sellar SFT or HPC and favorable visual outcome with the low risk of optic neuropathy.
Sellar SFT or HPC is a highly vascular and fibrous tumor and leads to partial resection. Fractionated gamma knife radiosurgery could provide both local tumor control and favorable visual outcome. Partial removal followed by fractionated gamma knife radiosurgery is one of the acceptable treatment strategies for patients with sellar SFT or HPC, especially older patients.
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. All authors who are members of The Japan Neurological Society (JNS) have registered online self-reported COI Disclosure Statement Forms through the JNS member website.