2022 年 9 巻 p. 95-100
2022 年 9 巻 p. 95-100
The coronavirus disease 2019 (COVID-19) pandemic continues to spread around the world, and widespread vaccination is considered the most effective way to end it. Although the efficacy of COVID-19 vaccines has been confirmed, their safety remains a concern. In this paper, we report two cases of ruptured vertebral artery dissecting aneurysm (VADA) immediately after messenger RNA (mRNA) anti-COVID-19 vaccination. In Case 1, a 60-year-old woman experienced sudden headache 3 weeks before her first dose of the Moderna mRNA-1273 COVID-19 vaccine. Magnetic resonance imaging showed dilatation of the right vertebral artery (VA) without intracranial hemorrhage. A day after the vaccination, she developed subarachnoid hemorrhage with pulmonary effusion due to a ruptured right VADA. She underwent endovascular internal trapping and parent artery occlusion under general anesthesia. In Case 2, a 72-year-old woman with a previous history of the left VA occlusion due to arterial dissection developed subarachnoid hemorrhage 7 days after the first dose of the Pfizer-BioNTech BNT162b2 COVID-19 mRNA vaccine due to a ruptured right VADA and underwent stent-assisted coil embolization under general anesthesia. The postoperative courses of these two cases were uneventful. The accumulation of more cases and further study are warranted to clarify the relationship between COVID-19 mRNA vaccination and ruptured intracranial dissecting aneurysms.
In response to the coronavirus disease 2019 (COVID-19) pandemic, multiple vaccines have been developed and distributed worldwide.1,2) Thus far, two main types of COVID-19 vaccines have been developed:3) adenoviral vectored vaccines (e.g., the Oxford-AstraZeneca AZD1222 (ChAdOx1) and the Johnson & Johnson JNJ-78436735 (Ad26.COV2 S) COVID-19 vaccines) and mRNA vaccines (e.g., the Pfizer/BioNTech BNT162b2 and the Moderna mRNA-1273 COVID-19 vaccines).4) The efficacy, immunogenicity, and safety have been shown to differ among these two types of vaccines.5) The efficacies of the adenovirus vector and mRNA vaccines in participants aged ≥18 years were shown to be 73% and 85%, respectively.5) Fever and fatigue were the most prevalent adverse effects reported in people receiving the adenovirus vector and mRNA vaccines, respectively.5) Several investigators have reported cerebrovascular diseases, including hemorrhagic stroke, in patients with COVID-19;6,7) however, cerebrovascular diseases after COVID-19 vaccination are rare. While some rare cases of vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis have been reported, mainly in association with viral vector vaccines,8) few reports have been published on cerebrovascular diseases after mRNA COVID-19 vaccination. In this paper, we report two cases of vertebral artery dissecting aneurysm (VADA) that ruptured immediately after mRNA anti-COVID-19 vaccination.
A 60-year-old woman with a previous history of dyslipidemia incidentally underwent magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) 10 years previously that showed no abnormality in the vertebral artery (VA) (Fig. 1A). She had no previous history of autoimmune or autoinflammatory diseases. Three weeks before the first dose of the Moderna mRNA-1273 COVID-19 mRNA vaccine, she experienced sudden headache and underwent MRI at a local clinic. Although MRA showed dilatation of the right VA (Fig. 1B), MRI showed no intracranial hemorrhage (Fig. 1C). She did not receive any medical care. She suddenly developed severe headache and dyspnea 1 day after the vaccination and was referred to our hospital. No neurological deficits other than slight disturbance of consciousness were identified. Blood tests were unremarkable with a normal platelet count, and her serum was negative for autoantibodies associated with vasculitis. Brain computed tomography (CT) showed massive subarachnoid hemorrhage (Fig. 1D), and chest CT revealed bilateral pulmonary effusion due to neurogenic cardiomyopathy (Fig. 1E). While the electrocardiogram showed negative T waves in V3-V6, the transthoracic echocardiogram showed mild hypokinesia of the apex without reduced ejection fraction. Three-dimensional CT angiography (3DCTA) of the head showed fusiform dilatation of the right VA, suggesting dissecting aneurysm (Fig. 1F). Since the posterior inferior cerebellar artery (PICA) originated from the extradural segment of the VA, we planned endovascular internal trapping and parent artery occlusion from the aneurysm to the right VA just distal to the origin of the PICA. The procedure was performed under general anesthesia (Fig. 2A). Both right and left VA angiography after internal trapping revealed that the dissecting lesion was not visualized (Fig. 2B and C). Postoperative MRI showed no evident complications of the internal trapping procedures, and the dissecting lesion of the right VA was not visualized on MRA (Fig. 2D). Her cardiopulmonary condition gradually improved, and she was discharged with a modified Rankin Scale 1.

(A) Magnetic resonance angiography (MRA) performed 10 years previously shows normal right vertebral artery (VA). (B) MRA performed 3 weeks before the vaccination shows dilatation of the right VA (arrowhead). (C) Fluid-attenuated inversion recovery magnetic resonance imaging (MRI) performed on the same day shows no intracranial hemorrhage. (D) Head computed tomography (CT) performed 1 day after the vaccination shows diffuse subarachnoid hemorrhage. (E) Chest CT performed on the same day shows bilateral pulmonary effusion. (F) Three-dimensional CT angiography (3DCTA) performed on the same day shows dissecting aneurysm in the right VA (arrowhead). The right posterior inferior cerebellar artery (PICA) originated from the extradural segment of the VA (arrow).

(A) Frontal view of the right vertebral artery angiography (VAG) before internal trapping shows dissecting aneurysm in the right VA. (B) Frontal view of the right VAG after internal trapping reveals that the dissecting lesion was occluded just distal to the origin of the right posterior inferior cerebellar artery (PICA). (C) Frontal view of the left VAG after internal trapping also reveals that the dissecting lesion was occluded. (D) Postoperative magnetic resonance angiography (MRA) performed 2 weeks after treatment reveals that the dissecting lesion of the right VA was not visualized.
A 72-year-old woman with a previous history of left VA occlusion due to arterial dissection accompanied by medullary infarction 8 years previously had been followed up with MRI every year. She had no previous history of autoimmune or autoinflammatory diseases. She developed pain at the injection site in her right upper arm after the first dose of the Pfizer-BioNTech BNT162b2 COVID-19 mRNA vaccine that gradually spread to her right shoulder and nape. Seven days after the vaccination, these pains graduated to severe occipital headache and she was referred to our hospital. She was fully conscious, and no neurological deficits were identified. Blood tests were unremarkable with a normal platelet count and her serum was negative for autoantibodies associated with vasculitis. Brain CT showed subarachnoid hemorrhage mainly in the posterior cranial fossa (Fig. 3A). 3DCTA showed a dissecting aneurysm with bleb-like protrusion on the right VA (Fig. 3B). Retrospectively, MRA performed 1 year previously showed caliber change of the right VA, suggesting that there had been a chronic arterial dissection (Fig. 3C). Since the origin of the right PICA was involved in the dissecting lesion and the contralateral VA had been occluded for 8 years, we planned stent-assisted coil embolization to keep the right PICA patent and maintain anterograde blood flow for posterior circulation via the right VA. The off-label use of intracranial stents was approved by our institutional ethical committee, and written informed consent was obtained before the procedure, which was performed under general anesthesia (Fig. 4A). Immediately before the procedure, 300 mg clopidogrel and 100 mg aspirin were orally administered. A 3.5 × 17 mm bladed stent (MicroVention Terumo, Tustin, CA, USA) was placed across the dissecting lesion, and detachable coils were deployed into the aneurysmal sac (Fig. 4B and C). Postoperative MRI showed no evident complications, and the dissecting aneurysm was not visualized on MRA (Fig. 4D). The patient was discharged with a modified Rankin Scale 1.

(A) Head computed tomography (CT) performed 7 days after vaccination shows massive subarachnoid hemorrhage in the posterior cranial fossa. (B) Three-dimensional CT angiography (3DCTA) performed on the same day shows a dissecting aneurysm with bleb-like protrusion on the right vertebral artery (VA). (C) Magnetic resonance angiography (MRA) performed 1 year previously shows caliber change of the right VA, retrospectively, suggesting chronic arterial dissection.
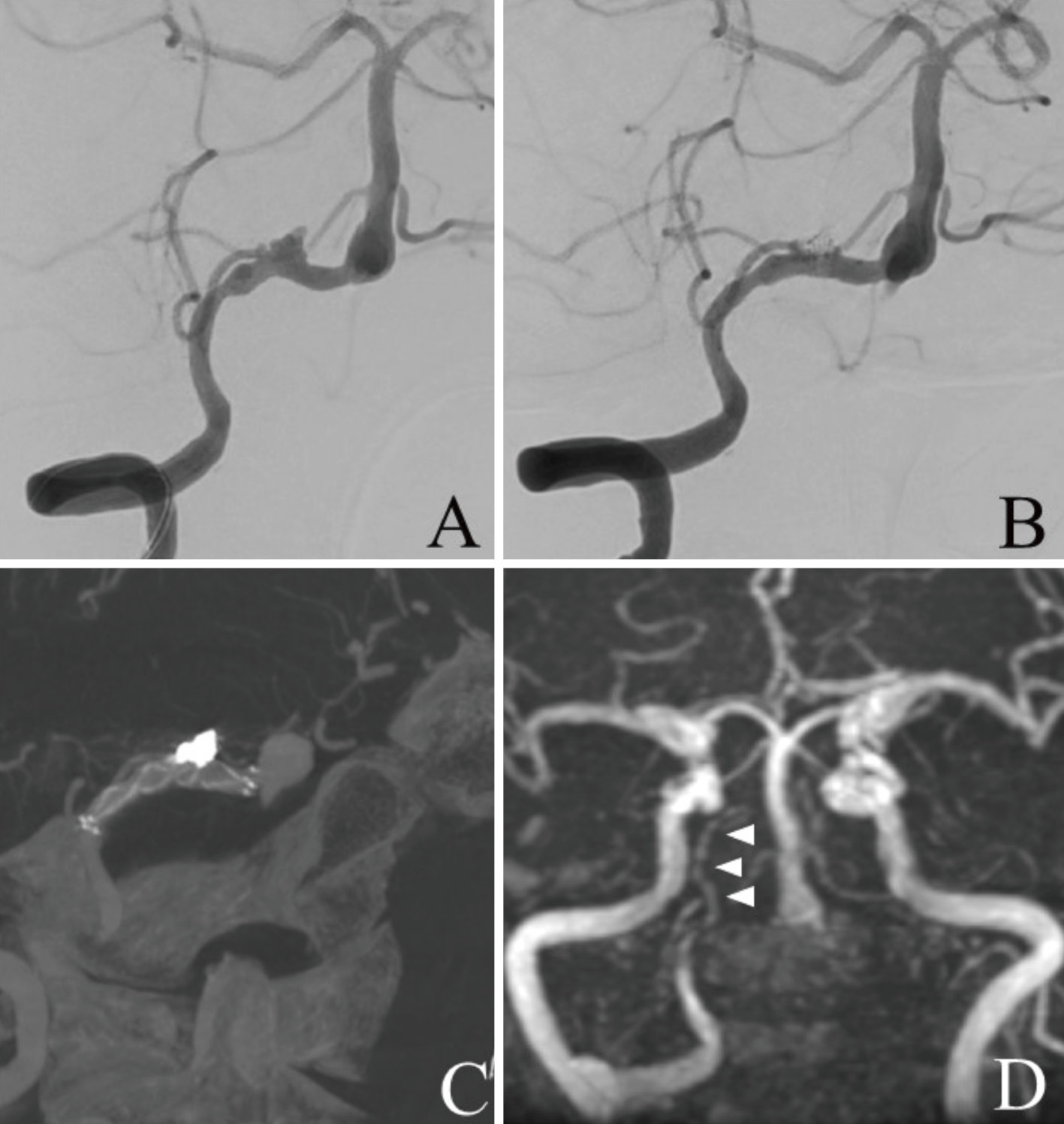
(A) Frontal view of the right vertebral artery angiography (VAG) before stent-assisted coil embolization shows dissecting aneurysm in the right vertebral artery (VA) involving the right posterior inferior cerebellar artery (PICA). (B) Frontal view of the right VAG after stent-assisted coil embolization shows coil occlusion of the aneurysmal sac and patency of the right VA and right PICA. (C) Cone beam computed tomography (CT) image shows the bladed stent placed across the dissecting lesion and coils deployed into the sac. (D) Postoperative magnetic resonance angiography (MRA) performed 2 weeks after treatment shows that the dissecting aneurysm is not visualized and the right PICA is patent.
Written informed consent was obtained from both of the patients and their next of kin prior to all procedures.
Here, we reported two cases of VADA that ruptured immediately after the administration of different mRNA anti-COVID-19 vaccines. In both cases, caliber irregularity of the VA was retrospectively identified on MRA before vaccination, suggesting that unruptured VA dissection had already developed. Then, these VADAs ruptured immediately after the vaccination. Several investigators have reported the natural course of unruptured intracranial arterial dissections (IADs). Mizutani9) reported that IAD rupture had occurred in only 1 of 93 patients with unruptured IADs during a mean follow-up of 3.44 years. Kobayashi et al.10) also reported that rupture of the VADA had occurred in only 1 of 113 patients with unruptured VADA during a mean follow-up of 2.9 years. Therefore, unruptured VADAs have low risk for bleeding at diagnosis; however, two successive ruptures of unruptured VADA occurred in the present cases. Inflammatory response in the saccular cerebral aneurysm wall, mainly in the form of infiltrating T cells and macrophages, is known to be associated with aneurysm rupture.11) There is also a report suggesting that local inflammation of the arterial wall spread by systemic inflammation could cause fragility and result in the rupture of cerebral arterial dissection.12)
COVID-19 mRNA vaccines, which have shown good efficacy and safety profiles in multiple clinical trials,13) have been widely administered. However, despite recent progress, mRNA vaccines may lead to a cascade of immunological events that can eventually result in the aberrant activation of the innate and acquired immune system.14) Before translation, mRNA vaccines can activate a number of proinflammatory pathways, including type I interferon and nuclear translation of nuclear factor (NF) -κB.14) The activation of these pathways forms the basis of immune-mediated diseases, especially in genetically predisposed individuals such as young females.14) Wadman15) also reported that the immune system response to lipids in the nanoparticle delivery vehicle of mRNA vaccines can cause short-term side effects due to the release of inflammatory mediators in the muscle. Indeed, rare cases of myocarditis and pericarditis within a few days after mRNA vaccinations have been reported.16) Moreover, not only autoimmune inflammatory disease but also non-autoimmune inflammatory disease following mRNA vaccination have been successively reported.17-19) Regarding Case 2 in the present report, the patient experienced pain at the injection site immediately after vaccination, which gradually spread to her nape; this could suggest that inflammation at the injection site extended to her nape, possibly to the wall of the preexisting VA dissection, possibly rupturing the aneurysm. Histological examination of the present cases was not done as they were treated with an endovascular procedure; therefore, there is no evidence that the vaccination evoked arterial wall inflammation or ruptured VADAs.
To the best of our knowledge, this study is the first to report ruptured VADAs after mRNA anti-COVID-19 vaccination. The accumulation of additional cases and further study are warranted to clarify the relationship between COVID-19 mRNA vaccines and ruptured intracranial dissecting aneurysms.
COVID-19: coronavirus disease 2019, CT: computed tomography, IAD: intracranial arterial dissection, mRNA: messenger RNA, MRI: magnetic resonance imaging, MRA: magnetic resonance angiography, NF: nuclear factor, 3DCTA: Three-dimensional CT angiography, VA: vertebral artery, VADA: vertebral artery dissecting aneurysm
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.