2022 年 9 巻 p. 295-299
2022 年 9 巻 p. 295-299
Arachnoiditis ossificans (AO) is a rare disease, wherein ossified lesions in the subarachnoid space obstruct the flow of spinal fluid or compress the spinal cord, thereby causing myelopathy. Here we describe a rare case of AO and discuss the diagnosis and treatment strategies for this disease. A 66-year-old man with a history of subarachnoid hemorrhage presented with gait disturbance and dysuria for 7 months. Spinal magnetic resonance imaging and computed tomography (CT) myelography showed syringomyelia at the T5-T8 level and dorsally tethered spinal cord at the T8-T10 level. Preoperative noncontrast CT was not performed. The patient was diagnosed with adhesive arachnoiditis and underwent arachnoidolysis. However, intraoperative findings showed the presence of ossification lesions on the dorsal surface of the spinal cord, and intraoperative ultrasound (IOU) showed a hyperintense lesion with acoustic shadowing on the dorsal surface of the spinal cord, with limited visibility of the spinal cord. After removal of the lesions, IOU showed untethered and well-decompressed spinal cord and restoration of cerebrospinal fluid pulsation. Based on these findings, the patient was finally diagnosed with AO, which is an extremely rare disease, with an unknown frequency of occurrence. Therefore, all patients with adhesive spinal arachnoiditis require a preoperative noncontrast CT scan to evaluate for ossification lesions. In this case, we were fortunate to be able to treat AO with IOU, which demonstrated specific findings.
A rare subtype of adhesive spinal arachnoiditis (SA), that is, arachnoiditis ossificans (AO), is considered a chronic inflammatory disease with the development of ossification of the arachnoid membrane.1-3) AO results in spinal cord compression or tethering, spinal cord edema, cerebrospinal fluid (CSF) flow blockage, and syringomyelia. These changes appear to be caused by a variety of causes, including previous surgery, trauma, spinal cord injury, infection, and subarachnoid hemorrhage.4) There have been limited case reports of AO in the past, and the exact incidence of the disease is still unknown. In this case report, we present a rare case of AO and discuss the diagnosis and treatment strategies for this disease.
A 66-year-old man with a history of subarachnoid hemorrhage (SAH) was referred to our hospital because of progressive gait disturbance and dysuria. He had undergone clipping surgery for vertebral artery dissection following SAH 12 years before. Postoperatively, he underwent ventriculoperitoneal (VP) shunting for hydrocephalus, and his perioperative course was complicated by a cerebellar infarction. After rehabilitation, the patient had residual mild paralysis of the right upper and lower limbs, with a good ability to walk without external aid. His condition had been stable for approximately 11 years thereafter; however, he started to develop gait and urinary problems seven months before his first visit in our hospital. His condition gradually worsened to the point where he needed a two-handed cane for ambulation. Spinal magnetic resonance imaging (MRI) and computed tomography (CT) myelography showed syringomyelia at the T5-T8 level and dorsally tethered spinal cord at the T8-T10 level (Fig. 1). Based on these findings, the patient was diagnosed with syringomyelia associated with SA, without adequate consideration of the presence of ossified lesions. Preoperative noncontrast CT was not performed because we determined that CT myelography could provide enough information on the dynamic CSF flow and the bony structures for surgical procedure. Spinal arachnoidolysis, untethering of the spinal cord, and duraplasty via T6-T9 expanding laminoplasty were planned.
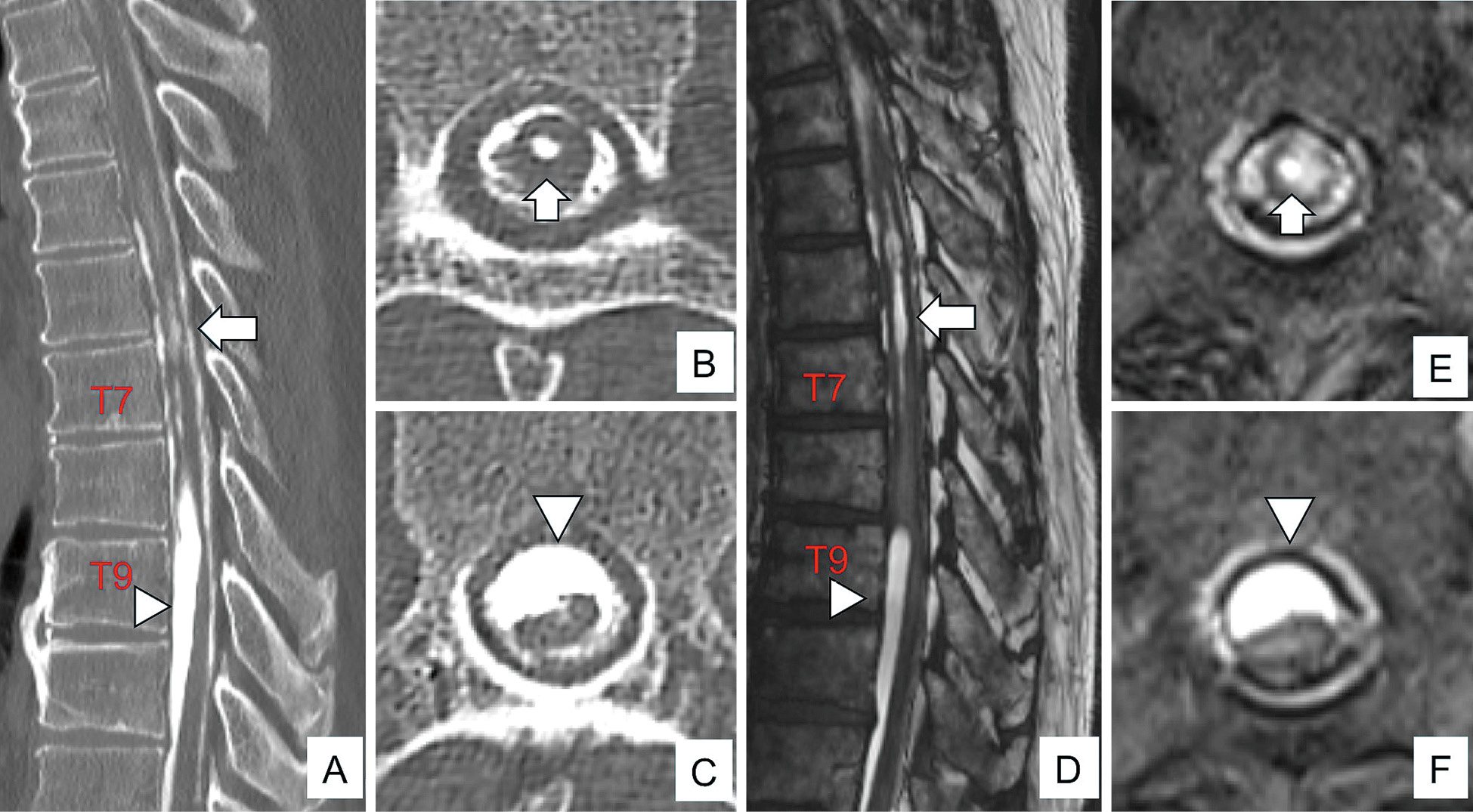
Preoperative computed tomography (CT) myelography and magnetic resonance imaging (MRI) images. (A-C) CT myelography showed syringomyelia (arrows) and dorsal shift of the spinal cord (arrowheads) due to arachnoiditis in the dorsal leptomeninges. (D-F) T2-weighted MRI without contrast showed syringomyelia (arrows) and a dorsal shift of the spinal cord (arrowheads) due to arachnoiditis in the dorsal leptomeninges. B, T7; C, T9; E, T7; and F, T9.
The patient was placed in a prone position under general anesthesia. Following the T6-T9 expanding laminoplasty, the dura mater was exposed. Intraoperative ultrasound (IOU) revealed a hyperechoic lesion with acoustic shadowing, which blocked the view of the spinal cord, on the dorsal surface of the spinal cord (Fig. 2A). Hard ossified tissue was visualized on the dorsal surface of the spinal cord when the dura mater was incised (Fig. 2B, C). The ossified lesion was adhered to the spinal cord and was carefully removed piece by piece as much as possible, without compressing the spinal cord. IOU showed further showed ossified lesions extending to the lateral of the spinal cord. They were also removed, and arachnoidolysis was additionally performed (Fig. 2D, E). At the final stage of surgery, the IOU showed a clear image of the untethered and well-decompressed spinal cord and restoration of CSF pulsation, while the syrinx of the spinal cord was unchanged (Fig. 2F). Pathological examinations revealed ossified tissue along with the bone marrow. A cluster of cells with round, nonatypical nuclei existed around the bone tissue (Fig. 3A). Postoperative CT scans showed a residual ossified lesion on the dorsal surface of the spinal cord at upper and lower levels outside the surgical field (Fig. 3B-D). Thus, the lesion was diagnosed with AO. Retrospective analysis of the CT myelography with reference to postoperative CT did not provide clues to differentiate the ossification lesions in the AO from the subarachnoid contrast agents. Postoperative MRI showed the disappearance of syringomyelia and successful untethering of the dorsally tethered spinal cord (Fig. 3E-G). Postoperatively, there were no surgical complications, and the patient's neurological symptoms remained stable. He has been receiving postoperative physiotherapy, and his ambulation ability has been improving.
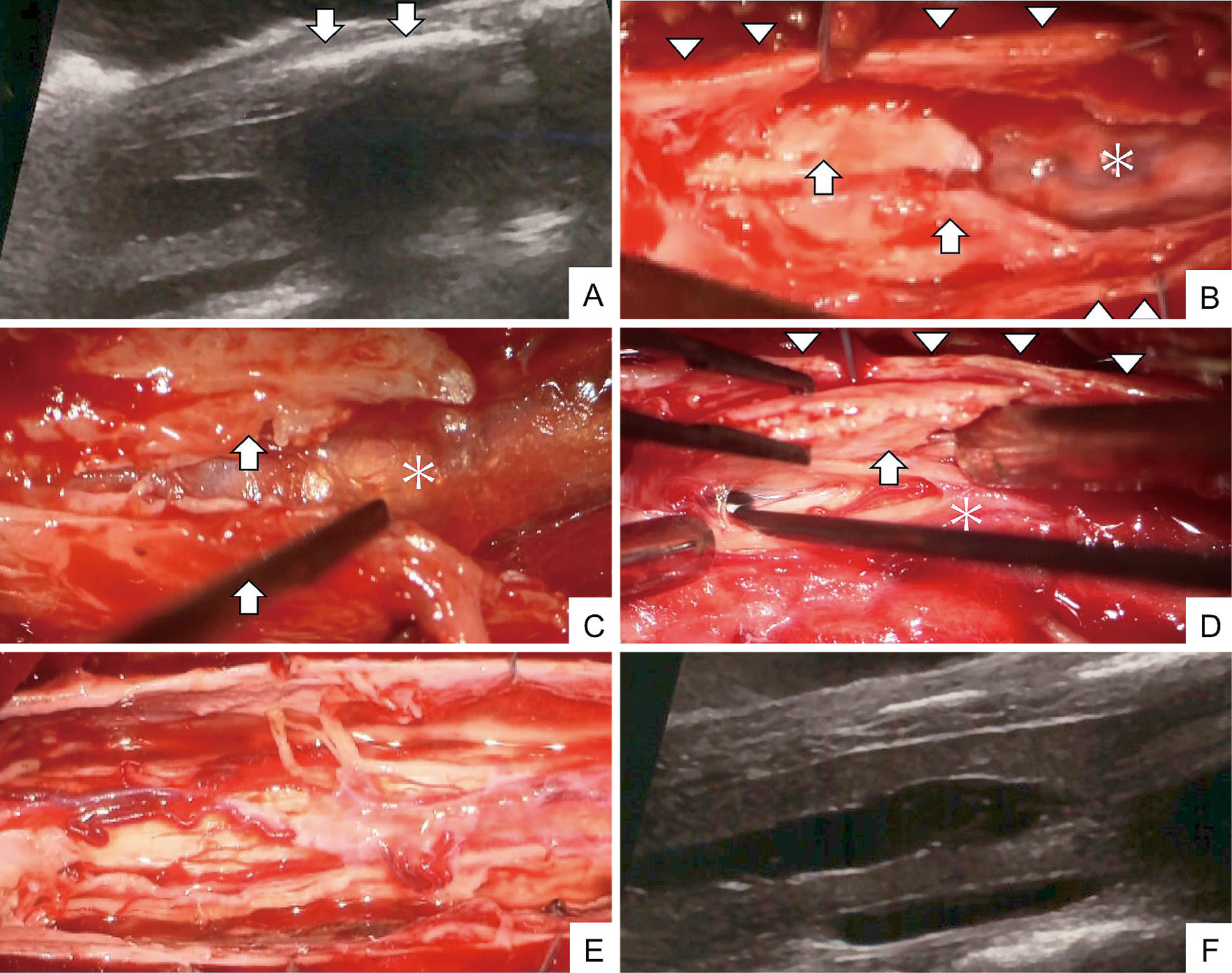
Surgical findings and intraoperative ultrasound images (Arrow, arachnoiditis ossificans; arrowhead, dura matter; asterisk, spinal cord). Intraoperative sagittal ultrasound image showing hyperintense structures with acoustic shadowing on the dorsal spinal cord (arrow). The spinal cord with syringomyelia can be partially identified (A). After opening the dura matter (arrowhead), the dorsal surface of the spinal cord was covered with arachnoiditis ossificans (arrow) (B-D). The ossified lesion was removed as far as possible without compressing the spinal cord (E). Then, the flow of spinal fluid can be detected by intraoperative sagittal ultrasound imaging (F).

Histopathological findings and postoperative computed tomography (CT) and magnetic resonance imaging (MRI) images. Histopathological examination shows ossified tissue along with the bone marrow tissue and scattered epithelial-like cells without atypia around the bone tissue (Hematoxylin & Eosin staining; original magnification: ×100) (A). T6-8 laminoplasty, T9 partial laminectomy, and removal of the arachnoiditis ossificans were performed. Postoperative CT shows calcified lesions on the dorsal spinal cord above and below the surgical levels (arrowheads) (B-D). T2-weighted MRI shows an improvement in syringomyelia and a dorsal shift of the spinal cord (E-G). B, T5; C, T7; E, T5; and F, T7.
Autopsy studies have often revealed that leptomeningeal calcifications, which are considered secondary to a degenerative process, are commonly encountered as small and asymptomatic lesions without subarachnoid adhesion.5) On the other hand, AO is a rare pathology, usually presenting with progressive myelopathy or cauda equina syndrome by compressing the spinal cord and cauda equina or blocking the CSF flow.1-3,6-12) There are limited small case series of AO, though some common characteristics have been reported. AO is related to chronic inflammation caused by trauma, surgical manipulation, or myelography. A history of subarachnoid hemorrhage, spinal trauma, infection, or oil-based spinal myelography is thought to influence the development of AO.11) The chronic inflammatory or noninflammatory stimuli are thought to induce the proliferative activity of the arachnoidal and osteoblastic cells.4) The thoracic and lumbar spine have been reported as common sites of occurrence but not the cervical spine.2,10) To the best of our knowledge, there have been four reports of AO after SAH, including our case, and all four cases involved the thoracic spine area (Table 1). There have also been reports in SA following SAH. Ishizaka et al. reported that the nine cases they reviewed all involved the thoracic spine.13) These results might be because the patient was bedridden for several weeks after the onset of SAH. Blood clots in the subarachnoid space usually accumulate in the midthoracic spine, which becomes the bottom of the spinal curvature in the supine position. Thus, the slow process of chronic inflammation may have led to the AO with obstruction of CSF flow over a period of more than 11 years in our case.
| Author, Year | Age/Sex | Location of AO | Symptoms |
|---|---|---|---|
| AO, arachnoiditis ossificans | |||
| Revilla TY20), 1999 | 68/F | T5-T9 | Spastic paraparesis and low back pain |
| Kahler RJ15), 2000 | 62/M | T2-11 | Tetraparesis and low back pain |
| Maulucci CM2), 2014 | 58/F | T6-T8 | Asymptomatic from AO |
| Our case | 66/M | T8-10 | Spastic paraparesis and urinary problems |
AO is most accurately diagnosed with noncontrast CT, which clearly depicts the ossified lesions in the arachnoid.11) Ossification lesions are described as hyperintensities on T1-weighted MRI (T1WI) and hyper- or hypointensities on T2WI.14) It may be difficult to make a correct diagnosis of AO with MRI alone if the ossified lesion is small. CT myelography can be a good alternative option to evaluate the spinal cord and subarachnoid space, especially when MRI cannot be feasible. Furthermore, it can provide valuable information on the dynamic CSF flow to help establish a surgical strategy. However, CT myelography can give misleading results of AO in the presence of contrast media, as described in the present case.11) Noncontrast CT scan, therefore, is essential for cases of SA in addition to CT myelography to detect ossifications of the arachnoid. Given that the incidence of AO is still unknown; all patients with SA should be carefully assessed on noncontrast CT, in consideration of the presence of ossifications.
The treatment of choice for AO is the removal of the ossified lesions and arachnoidolysis for associated SA. However, surgical outcomes are not always good because ossified lesions are frequently adherent to nerve tissues.2,6,11) Large ossified lesions; therefore, should be removed piece by piece instead of through an en bloc removal. Moreover, the location and lateral or ventral extension of ossified lesions influence the surgical outcomes. The surgery is challenging in cases with the entire spinal cord or the nerve roots of the cauda equina covered by ossifications.6,7,9,10) While it is unnecessary to remove all ossifications, those strongly compressing the spinal cord or obstructing CSF flow should be taken out by IOU. In cases that do not ensure adequate spinal fluid flow, insertion of a shunt into the syringomyelia associated with the AO could be another surgical option.15)
The usefulness of IOU has been proven in many spinal surgeries, while no report has been reported for AO to the best of our knowledge.16-19) In the present case, a hyperintense lesion was presented on the surface of the spinal cord, and the spinal cord could not be seen clearly. This phenomenon, called acoustic shadowing, occurs because the ossified lesion blocks most of the ultrasound wave and reflects it back to the probe. Acoustic shadowing in IOU is a specific finding for the diagnosis of AO as well as a good reference for intraoperative removal. In this case, IOU could allow intraoperative diagnosis of AO and intraoperative changing in the surgical plan. Finally, IOU clearly described the absence of any lesions compressing the spinal cord and the successful restoration of CSF pulsation. Thus, IOU is beneficial for determining which and how much ossified lesions should be removed.
AO is a rare type of SA, usually presenting with progressive myelopathy or cauda equina syndrome by compressing the spinal cord and cauda equina or blocking the CSF flow. As CT myelography can cause misleading results, a noncontrast CT scan should be performed preoperatively in all patients having SA. IOU is extremely useful in the surgical interventions for AO, a procedure consisting of the removal of ossification and arachnoidlysis. IOU can provide the images of acoustic shadowing suggestive of ossifications and a real-time evaluation of the removal of ossification.
Informed consent was obtained from the patient for the publication of this case report and the accompanying images.
The authors declare no conflicts of interest associated with this manuscript.