Article ID: 2014-0231
Article ID: 2014-0231
Thrombus formation in a pulmonary vein stump after pulmonary lobectomy is extremely rare, but can trigger cerebral embolism of unknown cause. We encountered a case of cerebral embolism in a 58-year-old man 2 days after left upper lobectomy. Since intravenous administration of recombinant tissue plasminogen activator was contraindicated, thrombus removal by endovascular reperfusion therapy was performed. Cerebral angiography showed left internal carotid artery occlusion. Thrombus removal using a retrieval device was performed and complete recanalization of the left internal carotid artery was obtained. Although blood abnormalities or arrhythmia such as atrial fibrillation were not observed, thrombus in the left upper pulmonary vein stump was detected with contrast-enhanced computed tomography of the body trunk, which was therefore considered as the source of cerebral embolism. The patient is continuing on anticoagulant therapy to prevent embolism recurrence caused by thrombus formation in the pulmonary vein resection stump. To the best of our knowledge, this is the first report of thrombus removal by acute-phase endovascular reperfusion therapy to treat cerebral embolism likely caused by thrombus formation in the pulmonary vein stump after left upper lobectomy. When cerebral embolism of unknown cause develops after left upper lobectomy, thrombus formation in the pulmonary vein stump should be considered among the differential diagnoses. For acute-phase onset of cerebral embolism after pulmonary lobectomy, thrombus removal by endovascular reperfusion therapy may be considered as one of the therapies.
Thrombus formation in a pulmonary vein stump after pulmonary lobectomy is extremely rare. Thrombi in the pulmonary veins are the most distantly situated among the upstream sources of arterial embolism, and can cause embolism in all types of tissues, including the brain, kidneys, spleen, and intestinal tract.1,2) Previously, pulmonary venous thrombosis has been suggested as one potential trigger for cerebral embolism of unknown cause.3) Cerebral embolism caused by thrombus formed in the pulmonary vein stump after pulmonary lobectomy is a rare complication, and the concept has only been recognized in recent years.2) Reports from authors specializing in the cerebrovascular system have not been seen previously. We describe herein the first description of experience with a case in which thrombus removal was performed by acute-phase endovascular reperfusion therapy to treat cerebral embolism attributed to thrombus formation in a pulmonary vein stump after left upper lobectomy.
The patient was a 58-year-old man who smoked 20 cigarettes a day for 23 years. He had previously undergone craniotomy and clipping to treat an unruptured cerebral aneurysm at the bifurcation of posterior communicating artery and left internal carotid artery at 55 years old. Medical examination showed an abnormal shadow in the left lung, and a 17-mm lesion was observed in the left upper lobe on chest computed tomography (CT). Transbronchial lung biopsy determined this to be adenocarcinoma. The patient subsequently underwent left upper lobectomy with video-assisted thoracoscopic surgery, with the left upper pulmonary vein cut off using a linear stapler at that time. Lymph node metastasis was not observed and the patient was determined to have stage IA early lung cancer. Although no issues were seen during the postoperative clinical course, the patient developed sudden disturbance of consciousness, right hemiplegia, and total aphasia 2 days postoperatively while in the hospital ward. Body temperature was 36.8°C, blood pressure was 122/78 mmHg, and heart rate was 84 beats/min without atrial fibrillation. Blood test findings showed no abnormalities in the complete blood count, with a prothrombin time of 12.9 s (normal range 10–12), activated partial thromboplastin time of 39.6 s (normal range 24–39), fibrinogen level of 605 mg/dl (normal range 200–400), D-dimer level of 1.89 μg/ml (normal range 0–0.99), and fibrin degradation product level of 6 μg/ml (normal range 0–5), demonstrating only mild activation of the coagulation system. Cardiogenic cerebral embolism was considered among the differential diagnoses, but brain natriuretic peptide level was 7.0 pg/ml (normal range 0–18.4), which was within the normal range. Protein C, protein S, and antithrombin III levels were likewise within the normal ranges. The patient was negative for lupus anticoagulant and anti-cardiolipin antibody, and did not exhibit other abnormalities in blood biochemical test results. Chest radiography did not show any enlargement of the superior mediastinal shadow or cardiac shadow. Left internal carotid artery occlusion and acute-phase cerebral infarction in the left middle cerebral artery region were observed on head magnetic resonance angiography (MRA) and diffusion-weighted imaging (DWI) conducted 30 min after onset, respectively (Fig. 1). Since this occurred 2 days after pulmonary lobectomy, we considered intravenous administration of recombinant tissue plasminogen activator (rt-PA) to be contraindicated, and therefore performed emergency thrombus removal by endovascular reperfusion therapy.
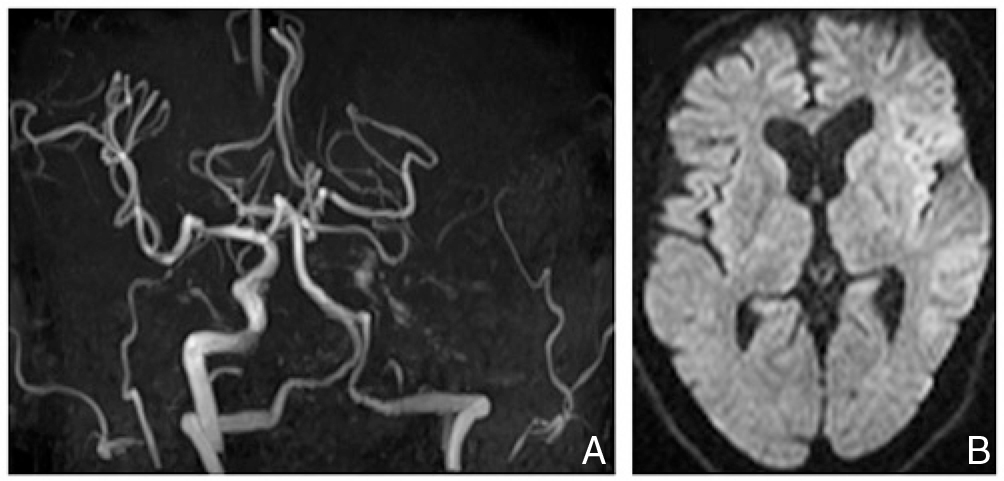
Head magnetic resonance imaging after onset. A: Magnetic resonance angiography demonstrating complete occlusion of the left internal carotid artery. B: Diffusion-weighted imaging showing subtle ischemic changes in the territory of the left middle cerebral artery.
Angiography was performed under local anesthesia. Left internal carotid artery images showed left internal carotid artery occlusion at the level of the posterior communicating artery bifurcation (Fig. 2A). Right internal carotid artery images showed that the left middle cerebral artery was not visualized via the anterior communicating artery, so the occlusion was considered to be located between the distal left internal carotid and proximal left middle cerebral arteries. Collateral blood flow to the territory of the left middle cerebral artery was not visualized at all. First, we performed aspiration from the proximal side of the occlusion using a Penumbra 5MAX reperfusion catheter (Penumbra, Alameda, California, USA), but only a small thrombus was able to be aspirated. We considered the occlusion to be caused by a relatively rigid thrombus; therefore, using a V2.5 Firm Merci retriever (Concentric Medical, Mountain view, California, USA), we were able to retrieve a relatively large thrombus. Left internal carotid artery imaging after thrombus removal showed complete recanalization of the left internal carotid artery, and visualization of the left middle cerebral artery periphery was also satisfactory (Fig. 2B). Three hours had passed from onset to recanalization of the left internal carotid artery.

Pre- and postoperative angiography. A: Preoperative left internal carotid angiography demonstrating internal carotid artery occlusion at the level of the origin of the posterior communicating artery. B: Left internal carotid angiography after thrombectomy with the Merci retrieval device, showing complete reperfusion of the internal carotid and middle cerebral arteries.
The symptoms of disturbed consciousness, right hemiplegia, and total aphasia did not improve after thrombus removal. To prevent embolism recurrence of unknown cause, anticoagulant therapy was started right after endovascular reperfusion therapy. Head MRA conducted the day after thrombus removal showed left internal carotid artery patency, but DWI showed extensive cerebral infarction in the territory of the left middle cerebral artery. We discontinued anticoagulant therapy for fear that the ischemic area might develop hemorrhagic transformation. Swelling without hemorrhage became more pronounced at the cerebral infarction site, and decompressive craniectomy was performed. Fourteen days of electrocardiographic monitoring conducted after thrombus removal did not show arrhythmias such as atrial fibrillation. Transthoracic echocardiography and venous ultrasonography of the lower limbs were conducted to search for the source of embolism, but did not show any abnormalities. Contrast-enhanced CT (CECT) of the trunk conducted 7 days after thrombus removal showed thrombus formation in the left upper pulmonary vein stump after left upper pulmonary lobectomy (Fig. 3A). Since no other lesions that may have caused the embolism were apparent, the source of embolism was determined to be thrombus in the pulmonary vein stump. In addition, acute splenic infarction was detected on CECT, and was thought to have developed at the same time as cerebral embolism (Fig. 3B). Anticoagulant therapy was restarted and continued in order to prevent the recurrence of embolism caused by thrombus formation in the pulmonary vein stump. Symptoms of right hemiplegia and aphasia remained, but the patient became able to feed himself, and was transferred to a rehabilitation hospital 27 days after the onset of cerebral infarction. As of the time of writing, the patient is continuing anticoagulant therapy.
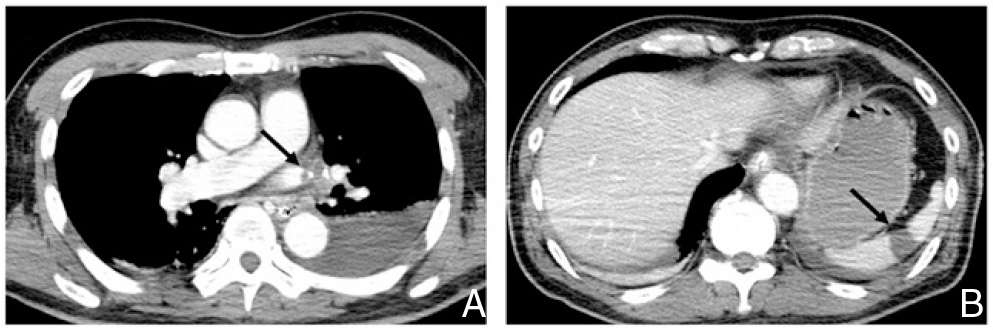
Contrast-enhanced computed tomography of the body trunk. A: Contrast-enhanced computed tomography of the chest demonstrating thrombus formation (arrow) in the pulmonary vein stump after left upper lobectomy. B: Contrast-enhanced computed tomography of the abdomen showing acute splenic infarction (arrow).
Thrombus formation in the pulmonary vein stump after pulmonary lobectomy is extremely rare, and only 13 cases have been reported to the best of our knowledge. These reports, including the present case, are listed in Table 1.4,5,6–11) In addition to these case reports, Ohtaka et al. conducted CECT screening within 2 years after pulmonary lobectomy at two separate institutions over several years, and separately reported 5 patients and 7 patients who developed thrombosis in a pulmonary vein stump from each institution.1,2) The majority of guidelines recommend plain CT or non-CT assessments for follow-up after pulmonary lobectomy.12) CECT is thus not performed postoperatively in many cases and thrombus in the pulmonary vein stump can be overlooked, which may lead to an underestimation of incidence.1,2) In fact, of the 12 patients reported by Ohtaka et al., only 1 patient developed cerebral embolism, indicating that many asymptomatic patients with thrombus formation may exist.1,2) Conversely, reports have described 4 patients treated for embolism of unknown cause in which thrombus in the pulmonary vein stump was not observed on imaging, even though embolism developed within several days after pulmonary lobectomy (Table 2).13–15) The events in those patients occurred in the acute phase after pulmonary lobectomy and thrombus formation may have occurred in the pulmonary vein stump, but were not detected by CECT. As indicated above, there may be cases in which the thrombus is not observed even if embolism occurs, and there may be even more cases where thrombus in the pulmonary vein stump is overlooked. The 26 patients, including the present case, who showed thrombus formation in the pulmonary vein stump after pulmonary lobectomy, and the 4 patients with suspected thrombus formation had all undergone left upper lobectomy. Post-left upper lobectomy is reportedly a significant risk factor for thrombus formation within the pulmonary vein stump.1,2) For anatomical reasons, the left upper pulmonary vein stump is considered to be significantly longer than other pulmonary vein stumps.2,8) Dividing the left upper pulmonary vein to the pericardium may shorten the stump, but actually this surgical technique does not seem practical for all patients because of the complicated and invasive nature.2,8) Turbulence and stagnation may thus be more likely to occur within this long pulmonary vein stump, resulting in a higher risk of thrombus development.1) The pulmonary veins are the most distantly situated of all upstream sources of arterial embolism, and pulmonary vein thrombus can cause embolism in all types of tissues. We describe schematic illustration of this etiology in Fig. 4. The frequencies of thrombus formation in the pulmonary vein stump after pulmonary lobectomy are reported as 3.3% and 3.6% in all patients who have undergone lobectomy.1,2) To date, no reports have described thrombus formation in the stump of a pulmonary vein other than the left upper pulmonary vein. When limiting the data to post-left upper lobectomy, the reported frequencies of thrombus formation in the pulmonary vein stump are relatively high, at 3.4%, 13.5%, and 17.9%.1,2,10) For this reason, it must be kept in mind that embolism may develop due to thrombus formation in the pulmonary vein stump after left upper lobectomy.
| Authors & Year | Age & Sex | Location of lobectomy | Postoperative period | Location of embolism | Symptoms | Treatment |
|---|---|---|---|---|---|---|
| Seki et al. (1989)11 | 75, M | Left upper | 6 months | Lower extremity | Lower limb pain | Surgical excision of thrombi in the pulmonary vein stump and the external iliac artery |
| Schwalm et al. (2004)9 | 73, M | Left upper | 20 days | Brain | Transient ischemic attack | Not reported |
| Nagaoka et al. (2008)7 | 76, M | Left upper | 13 months | Kidney | Flank pain | Anticoagulation therapy |
| Ohtaka et al. (2012)8 | 64, F | Left upper | 19 months | No | Asymptomatic | Anticoagulation therapy |
| 66, M | Left upper | 18 months | Brain | Not reported | Conservative | |
| 53, F | Left upper | 2 months | No | Asymptomatic | Anticoagulation therapy | |
| Ichimura et al. (2013)10 | 61, F | Left upper | 4 days | Kidney | Abdominal pain | Anticoagulation therapy |
| 70, M | Left upper | 24 months | No | Asymptomatic | Anticoagulation therapy | |
| 76, M | Left upper | 3 months | No | Asymptomatic | Anticoagulation therapy | |
| 71, M | Left upper | 3 months | No | Asymptomatic | Anticoagulation therapy | |
| Ohira et al. (2013)6 | 46, F | Left upper | 6 months | Brain | Consciousness disorder | Surgical excision of thrombus in the pulmonary vein stump, anticoagulation therapy |
| Asai et al. (2013)5 | 76, F | Left upper | 2 days | Suspected brain | Transient syncope | Anticoagulation therapy |
| Gual-Capllonch et al. (2013)4 | 70, M | Left upper | 7 years | Brain | Motor aphasia | Anticoagulation therapy |
| Present case (2013) | 58, M | Left upper | 2 days | Brain, spleen | Consciousness disorder, hemiplegia, aphasia | Endovascular removal of thrombus in the internal carotid artery, anticoagulation therapy |
| Authors & Year | Age & Sex | Location of lobectomy | Postoperative period | Location of embolism | Symptoms | Treatment |
|---|---|---|---|---|---|---|
| Oura et al. (2005)14 | 70, M | Left upper | 4 days | Kidney | Abdominal pain | Conservative |
| 70, F | Left upper | 4 days | Spleen | Abdominal pain | Conservative | |
| Asteriou et al. (2010)13 | 53, F | Left upper | 3 days | Kidney | Flank pain | Anticoagulation therapy |
| Tamaki et al. (2013)15 | 68, M | Left upper | 4 days | Kidney, lower extremity | Back pain, lower limb pain | Anticoagulation therapy |

Schema of cross section of the heart from the front showing the etiology of embolism caused by thrombus in the pulmonary vein (PV) stump after left upper pulmonary lobectomy. Cut off (straight line) of a LUPV forms a left upper PV stump during left upper pulmonary lobectomy. Turbulence and stagnation of blood occur within this long PV stump, resulting in thrombus development. Such PV thrombus can cause embolism in all types of tissues (dotted arrow). LLPV: left lower PV; LUPV: left upper PV; RLPV: right lower PV; RUPV: right upper PV.
A wide range of reports have examined the development of embolism due to thrombus in the pulmonary vein stump, reporting intervals from 2 days to 7 years after pulmonary lobectomy.4,5) When delayed embolisms develop in patients after pulmonary lobectomy, the condition may not be well recognized as a post-pulmonary lobectomy complication. As shown in the 4 patients in Table 2, even if thrombus is not observed in the pulmonary vein stump, thrombus formation in the pulmonary vein stump is suspected when symptomatic embolism develops within several days after left upper lobectomy. In the present case, the patient developed cerebral embolism 2 days after pulmonary lobectomy, strongly suggesting an association between embolism and surgical treatment. For this reason, thrombus formation in the pulmonary vein stump was suspected, and body trunk CECT was performed to identify the source of embolism and to screen for non-cerebral embolism.
No previous reports have described thrombus removal by endovascular reperfusion therapy to treat acute-phase cerebral embolism caused by thrombus in the pulmonary vein stump, as demonstrated in the present case. In acute-phase cerebral embolism after pulmonary lobectomy, intravenous administration of rt-PA is contraindicated. Thrombus removal by endovascular reperfusion therapy may therefore be considered as one of the therapies, as shown in the present case. To detect thrombus promptly as quickly as possible before infarction, routine CECT for patients after left upper pulmonary lobectomy has been recommended.2,8) If thrombus formation in the pulmonary vein stump is observed, anticoagulant therapy should be immediately initiated to prevent future embolism.1) In many cases, anticoagulant therapy is indeed performed, resulting in the disappearance of thrombus from the pulmonary vein stump and the prevention of embolism development after anticoagulant therapy.2,4–8,10) Ichimura et al. indicated that in patients whose thrombus disappeared after 6 months of anticoagulant therapy, thrombus formation did not reappear even though anticoagulant therapy was subsequently discontinued, and thus proposed 6 months of anticoagulant therapy as sufficient.10) However, as shown by Ohtaka et al., cases of newly formed thrombus in the pulmonary vein stump may arise even if thrombus is not observed with CECT 6 months previously.8) Therefore, thrombus formation may reoccur in the pulmonary vein stump with the discontinuation of anticoagulant therapy. Gual-Capllonch et al. reported a patient who developed embolism 7 years after pulmonary lobectomy, indicating that absence of embolism development cannot be verified without long-term follow-up.4) Clear guidelines are lacking on how long anticoagulant therapy should be given. In the future, further investigation with accumulation of case reports appears warranted.
This is the first report to describe thrombus removal by endovascular reperfusion therapy during the acute phase to treat cerebral embolism that was attributed to thrombus formation in the pulmonary vein stump after left upper lobectomy. When cerebral embolism of unknown cause develops after left upper lobectomy, thrombus formation in the pulmonary vein stump should be considered among the differential diagnoses. For acute-phase onset of cerebral embolism after pulmonary lobectomy, thrombus removal by endovascular reperfusion therapy may be considered as one of the therapies.
The authors thank Dr. Yoshihiro Miyamoto, Dr. Tomofumi Hirose, Dr. Rei Enatsu, Dr. Yasuzumi Matsui, and Dr. Naoki Matsumoto for their support in patient care.
The authors have no personal, financial, or institutional interest in any of drugs, materials, or devices in the article. All authors who are members of The Japan Neurosurgical Society (JNS) have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.