論文ID: cr.2014-0077
論文ID: cr.2014-0077
This report presents rosette-forming glioneuronal tumor (RGNT) of the tectum in a 24-year-old woman in whom spontaneous disappearance of contrast enhancement (CE) on magnetic resonance (MR) imaging was observed during 9-year follow-up period before therapeutic intervention. MR imaging obtained 9 years ago when she first visited local hospital with headaches showed a mass of the brain stem with CE. Follow-up MR imaging showed disappearance of CE without tumor growth. Nine years later, she was admitted to our hospital with headache and nausea, due to obstructive hydrocephalus. She underwent endoscopic third ventriculostomy (ETV) and tumor biopsy. Histological study revealed RGNT. To our knowledge, this is the first report presenting that the RGNT may show spontaneous disappearance of CE without tumor growth. It is unclear what this phenomenon means, however, knowledge of this phenomenon may be helpful for correct diagnosis and for follow up of RGNT.
Rosette-forming glioneuronal tumor (RGNT) of the fourth ventricle is a mixed glioneuronal neoplasm recently described in the World Health Organization (WHO) Classification of Central Nervous System (CNS) Tumors in 2007.1) It is characterized by distinctive histological appearances of neurocytic rosettes with a pilocytic astrocytic component. These tumors have been reported as having a slow progression and favorable outcome with little risk of recurrence by surgical excision without adjuvant therapy. In this article, we present an RGNT of the tectum in a 24-year-old woman in whom spontaneous disappearance of contrast enhancement (CE) on magnetic resonance (MR) imaging was observed during 9-year follow-up period before therapeutic interventions.
A 24-year-old Turkish woman experienced headaches 9 years ago and MR imaging performed in Turkey showed a mass of the brain stem with CE (Fig. 1a). Her symptom was relieved spontaneously. Scheduled MR imaging follow up was performed afterwards. During this follow-up period, the MR imaging showed spontaneous disappearance of CE without enlargement of the tumor (Fig. 1b, c). This woman, now living in Japan, was admitted to our hospital with headache and nausea in May 2011. During the previous week, she experienced paroxysmal headache and nausea several times a day. Physical examination, including a neurological examination, found no abnormalities. Computed tomography (CT) scan showed hydrocephalus and low density mass at midbrain (Fig. 2a). MR imaging performed at our hospital showed enlargement of midbrain lesion without CE together with severe stenosis or obstruction of aqueduct (Fig. 2b–f). With diagnosis of low grade glioma causing obstructive hydrocephalus, and with some possibility of demyelinating disease, inflammatory disease, or parasitic infectious disease, she underwent endoscopic third ventriculostomy (ETV) and tumor biopsy. Histology of the tumor biopsy revealed biphasic morphology; a neurocytic and an astrocytic component (Fig. 3a–d). The neurocytic component consisted of neurocytic rosettes and perivascular rosettes with positive staining for synaptophysin (SYN) and neurofilament protein (NFP). The astrocytic component intervened rosettes and was positive for glial fibrillary acidic protein (GFAP) and oligodendrocyte transcription factor 2 (Olig2). Ki-67 labeling index was low at 1.1%. An RGNT was diagnosed. Her post-operative course was uneventful, and size of ventricle diminished on postoperative CT scan. She has been followed for 17 months postoperatively without any clinical worsening or tumor enlargement.
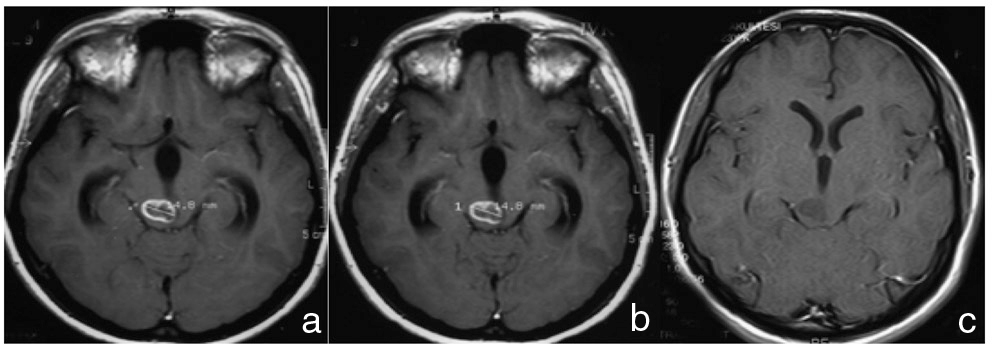
Magnetic resonance (MR) imaging performed previously. Gadolinium-contrasted T1-weighted MR imaging at first time (a) showing hypointense mass with contrast enhancement of midbrain in 2002. Follow-up MR imaging 3 months later (b) showing no change. Follow-up MR imaging 9 months after first MR imaging (c) showing spontaneous disappearance of contrast enhancement without tumor growth.

Computed tomography (CT) scan and magnetic resonance (MR) imaging performed in 2011 at her first visit to our hospital. CT scan (a) showing hydrocephalus and low intensity mass at midbrain. T1-weighted MR imaging (b) revealing the enlargement of tumor. Gadolinium-contrasted T1-weighted MR imaging (c) showing no contrast enhancement. T2-weighted MR imaging (d) showing high intensity lesion. Diffusion weighted imaging (e) showing iso-intensity lesion. MR spectroscopy (f) showing elevation of chorine (Cho) and no remarkable descent of N-acetyl-L-aspartate (NAA). Cre: creatine.

Photomicrographs demonstrating histological staining of tumor specimens. Hematoxylin and eosin staining (a–c) showing biphasic morphology; a neurocytic component presenting rosette-forming and an astrocytic component intervening the rosettes structure. Immunohistochemical staining (d). Neurocytic component is positive for synaptophysin (SYN) and neurofilament protein (NFP), an astrocytic component is positive for glial fibrillary acidic protein (GFAP) and oligodendrocyte transcription factor 2 (Olig2), and Ki-67 labeling index is low at 1.1%. Original magnification ×200 (a) and ×400 (b–d).
RGNT is classified as a WHO grade I tumor, which was first reported as a dysembryoplastic neuroepithelial tumor of the cerebellum by Kuchelmeister et al.,2) and described as a new entity by Komori et al. in 2002.3) Finally, it was described in the WHO Classification of CNS Tumors in 2007.1) To date, about 70 cases of RGNT have been published in the medical literature. The tumor was described as “a rare, slowly growing neoplasm of the fourth ventricular region, preferentially affecting young adults and composed of two distinct histological components, one with uniform neurocytes forming rosettes and/or perivascular pseudorosettes, the other being astrocytic in nature and resembling pilocytic astrocytoma (PA).” In general, typical RGNT appearances on MR imaging scan have been described as iso/hypointense on T1-weighted and hyperintense on T2-weighted sequences. The tumor was solid and/or cystic. Some enhancement is observed in 69.2% of the cases, and calcification was noted in 21.2% of the cases.4,5) In our case, it was comparable to RGNT in terms of patient characteristic, clinical course suggesting non malignancy, location of the tumor, MR imaging, and histopathological findings. On the other hand, the present case has showed fluctuation of CE.
To the best of our knowledge, our case is the first report mentioning that RGNT may show spontaneous disappearance of CE without tumor growth. Because the disease is relatively rare and the number of reported cases is limited, natural course and change in radiological images during follow up of RGNT has not been well characterized. Although, it is not clear what these phenomenon means, at least, in our case, clinical course did not worsen or tumor did not enlarge before and after CE fluctuations.
Spontaneous fluctuations of CE of PA has been reported, and CE changes were imaged in 12/39 (30.8%) patients with PA.6) This change in CE happened without change in tumor size or MR imaging signal intensity. It is already known that the degree of CE at the time of radiological diagnosis is unreliable in predicting clinical behavior of PA.7) Therefore, in our opinion, RGNT consisting of astrocytic cells with pilocytic morphology may have potential of showing a similar phenomenon. Histopathological features of contrast enhancement portion is not clear, however, one reported tumor cells of enhancement portion had astrocytic components in case of spinal cord RGNT.8) Since appearance of CE often indicates malignant transformation of low grade gliomas, it is important to know this CE fluctuation in the follow up of RGNT.
In conclusion, RGNT shows spontaneous fluctuations including disappearance of CE occasionally. It is unclear what this phenomenon means, however, knowledge of this phenomenon may be helpful for correct diagnosis and for follow up of RGNT. Because natural course and fluctuations of CE of RGNT has not been well clarified, further examination including careful long-term follow-up studies and data gathering are needed for the better understanding of RGNT.
There is no disclosure of funding as no financial support or grants supported this article.
No author has any personal or institutional financial interest in the drugs, materials, or devices described in this article.