2016 Volume 92 Issue 2 Pages 56-68
2016 Volume 92 Issue 2 Pages 56-68
Neddylation is a reversible post-translational modification in which a small ubiquitin-like molecule called NEDD8 covalently binds to substrate proteins. Although a recent study suggests that neddylation is essential for formation and maintenance of dendritic spines in the brain, the role of this protein modification in the peripheral nerves is wholly unknown. In this study, we demonstrate that neddylation-related molecules, NEDD8 and DCUN1D2 (defective in cullin neddylation 1, domain containing 2), were concentrated at the paranode of peripheral myelin, in addition to the myelinated and unmyelinated Schwann cell bodies. These proteins were localized mainly within larger fibers, but not in fibers with small diameters. Developmental analyses showed that these molecules first appeared at the paranode during later stages of myelination, and this characteristic distribution disappeared in sulfatide-deficient mice in which paranodal axo-glial junctions were disrupted. These results suggest that the myelin paranode may be one of the regions where neddylation occurs within the peripheral nerves.
Myelinated axons are organized into a series of distinct subdomains for rapid and efficient action potential propagation by saltatory conduction.1) These domains, including the nodes of Ranvier, the adjoining paranodal regions, the juxtaparanodes, and the internodes, are formed by the interaction between axons and glia in both the central and peripheral nervous systems (CNS and PNS, respectively). In the paranodal regions, paranodal myelin loops bind to the axolemma and form paranodal axo-glial junctions. In development, the paranodal axo-glial junctions appear relatively late during myelination, as they are first generated closer to the nodes by the outermost paranodal loop, then additional loops attach sequentially to the axonal membranes.2) A complex of two axonal cell adhesion molecules, contactin and contactin-associated protein (Caspr), and the glial 155 kDa isoform of neurofascin (NF155) are involved in the formation and maintenance of this junction.3)–6) The proposed function of the paranodal axo-glial junction was to insulate the electrical activity generated at the nodes of Ranvier and to serve as a barrier that limits the lateral diffusion of axolemmal proteins, including voltage-gated ion channels, to maintain domain structure.7),8) In addition to the axo-glial junction, the myelin side of the paranode shows unique structure with characteristic molecular composition and its own function. E-cadherin and connexin32 are found at the myelin paranode, both of which form autojunctions to connect the paranodal loop of each layer. In addition, myelin-associated glycoprotein is concentrated at the myelin paranode in the PNS.9) In the PNS, paranodal myelin loops contain cytoplasm that directly connects the Schwann cell body with the inner or outer mesaxons. Thus, the paranodal region of myelin is believed to have an important role in molecular transport between the Schwann cell body and compact or deep layers of myelin.10)
Mice generated with a disruption in the gene encoding cerebroside sulfotransferase (CST; EC 2.8.2.11) show a lack of sulfatide, which is one of the major glycolipids in the myelin sheaths of the CNS and PNS. These mice also display disruption of the paranodal axo–glial junctions in both the CNS and PNS.11)–13) Altered localization of nodal proteins, including Na+ channels and paranodal proteins such as Caspr and NF155 were observed.12)–14) PNS axons of CST knockout (CSTKO) mice frequently contain degenerated mitochondria in the nodes of Ranvier.13) Similar changes were reported in mice with other paranodal abnormalities,15),16) suggesting that the paranodal axo-glial junction may be important not only for rapid conduction but also for axonal homeostasis. Thus, CSTKO mice are a useful tool for examining the influence of the paranodal junction on the localization of functional proteins in both the axons and myelin.
Neddylation is a reversible post-translational protein modification whereby a small ubiquitin like protein (UBL), NEDD8 (neural precursor cell expressed developmentally downregulated protein 8), is attached to substrate proteins through a series of enzymatic reactions.17) Activation of NEDD8 requires ATP.18) Neddylation is thought to be involved in the regulation of cellular homeostasis including transcriptional regulation and signaling pathways.17) This functional diversity depends on substrate proteins, including a group of proteins involved in transcription, DNA repair and replication, cell cycle regulation and chromatin organization, and remodeling.19) The well-characterized targets of neddylation are cullins. They serve as essential components of cullin-based E3 ubiquitin ligases. Members of the DCNL family (defective in cullin neddylation protein 1-like proteins) are the main regulators of neddylation20) which act by increasing the kinetic efficiency of the neddylation reaction for cullin.21),22) There are five DCNL proteins in human, mouse and rat termed DCUN1D1 to DCUN1D5 (defective in cullin neddylation 1, domain containing 1–5; also named DCNL1-5).23),24) DCUN1D1, 2, and 3 interact with cullins to modulate their neddylation in a nonredundant manner and regulate activation of cullin-RING-ligases, the largest family of E3 ubiquitin ligases in mammals.23) DCUN1D2 is an approximately 30 kDa protein, structurally characterized by a C-terminal potentiating neddylation domain (PONY) and a predicted amino-terminal ubiquitin-associated (UBA) domain, which directly binds to ubiquitin.20),23)
NEDD8 was originally identified as a highly expressed molecule in the embryonic brain.25) However, a recent report demonstrated that neddylation was increased during postnatal brain development, and this active post-translational modification in the synapse was shown to regulate the maturation, stability and function of dendritic spines in the brain.26) However, the distribution and function of neddylation-related molecules, including NEDD8 and DCUN1D2, in the PNS are entirely unknown.
In the present study, we examined the localization of DCUN1D2 and NEDD8 in the sciatic nerves, and found that these neddylation-related molecules were present mainly in the myelin paranodal regions and Schwann cell bodies. Accumulation of these proteins depends on fiber size, and was prominent in the myelin of larger fibers. Developmental analyses demonstrated that their accumulation at the paranodal regions appeared during late-stage myelin formation, and was affected in CST-deficient mice in which paranodal junctional formation was incomplete. These results suggest that the myelin paranode may be one of the regions where neddylation occurs, and this is influenced by paranodal junction formation and by the diameter of the nerves.
8-week-old and pregnant Wistar rats were purchased from Japan SLC (Hamamatsu, Japan). CST-deficient mice, maintained on a C57BL/6J background, were kindly provided by Dr. Koichi Honke (Kochi University Medical School, Nankoku, Japan). Genotypes were determined by PCR as previously described.11) The mouse line was maintained in the animal facility at the Tokyo University of Pharmacy and Life Sciences under University Guidelines for Care and Use of Animals. The experiments were performed in compliance with the Tokyo University of Pharmacy and Life Sciences Animal Use Committee guidelines on the care and use of animals.
Antibodies.Primary antibodies for Western blotting were: anti-C-terminus of DCUN1D2 rabbit IgG (1:200; GeneTex, San Antonio, TX) and anti-N-terminus of DCUN1D2 rabbit IgG (1:200; Aviva Systems Biology, San Diego, CA). Horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:10,000) was purchased from Jackson Immunoresearch (West Grove, PA).
Primary antibodies for immunostaining of teased nerves were: anti-C-terminus of DCUN1D2 rabbit IgG (1:200), anti-N-terminus of DCUN1D2 rabbit IgG (1:200), anti-E-cadherin rat monoclonal (1:200, Takara Biotechnology, Kyoto, Japan), anti-pan Na+ channel mouse monoclonal (1:1000, Sigma, St. Louis, MO), anti-myelin-associated glycoprotein (MAG) mouse monoclonal (1:200, Millipore Bioscience Research Reagents, Bedford, MA), anti-glial fibrillary acidic protein (GFAP) mouse monoclonal (1:200, Sigma, St. Louis, MO) and anti-NEDD8 rabbit IgG (1:200; Cell Signaling, Danvers, MA). Alexa Fluor® 488- or 594-conjugated species-specific secondary antibodies were purchased from Molecular Probes/Life Technologies (Carlsbad, CA) and used at a dilution of 1:3000.
Preparation of rat whole brain and sciatic nerve homogenates.Homogenates were prepared from 8-week-old male Wistar rat brains and sciatic nerves as previously described.27),28) Brain homogenate was prepared from five rat brains, and sciatic nerve homogenate was prepared from the nerves of 10 rats. All procedures were carried out on ice or at 4 °C. Briefly, brains were homogenized in 9 volumes (wt/vol) of homogenization buffer [0.32 M sucrose, 5 mM Tris-HCl, pH 7.5, 2 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′ tetraacetic acid (EGTA), 0.75 µM aprotinin, 1 µM leupeptin, 1 µM pepstatin, and 0.4 mM phenylmethylsulfonyl fluoride]. The homogenate was centrifuged at 1,000 g for 10 min (RPR-20; Hitachi Koki, Tokyo, Japan) to remove nuclei, and the supernatant was used as the brain homogenate. Sciatic nerves were snap frozen in liquid nitrogen. The frozen tissues were then ground to a powder (mortar/pestle) and homogenized in 9 volumes (wt/vol) of homogenization buffer. The homogenate was centrifuged at 500 g for 10 min to remove nuclei, and the supernatant was used as the sciatic nerve homogenate. To separate membranous and cytosolic fractions, the sciatic nerve homogenate was centrifuged (100,000 g for 35 min, Hitachi himac CP80α). The precipitated membranes were resuspended in a homogenization buffer. All prepared homogenates were stored at −80 °C. Protein concentrations were determined by using a bicinchoninic acid (BCA) assay (Pierce Biotechnology, Thermo Scientific, Rockford, IL).
Western blot analysis.Western blotting was performed as previously described27) with minor modifications. Briefly, each sample was separated by 10.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (GE Healthcare UK, Buckinghamshire, UK). The membranes were incubated for 30 min with blocking buffer containing 5% skim milk in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.1% Tween 20 (T-TBS) at room temperature. Subsequently, the membranes were incubated for 30 min with primary antibodies diluted in blocking buffer and washed three times in T-TBS prior to incubation for 30 min with secondary antibody in T-TBS. After washing three times in T-TBS, immunoreactivity was detected using an ECL system (GE Healthcare UK, Buckinghamshire, UK).
Preparation of teased nerve fibers.Sciatic nerves were removed from 10-week-old C57BL/6J and CST-deficient mice and Wister rats of various ages, including postnatal day 0 (P0), P1, P7, P14, P21 and 8-week-old rats. Freshly isolated sciatic nerves were teased apart on MAS-coated glass slides (Matsunami, Osaka, Japan) using 27G needles. The slides were air-dried, and stored frozen at −20 °C. For staining of DCUN1D2 C-terminus and MAG, teased fibers were fixed for 30 min at 4 °C with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), pH 7.4, then washed twice in PBS prior to immunofluorescence staining.
Immunofluorescence.Immunohistochemistry was performed as previously described29) with minor modifications. Briefly, the teased nerve fibers were incubated with 5% bovine serum albumin (BSA) in 0.1 M PB, pH 7.5, and containing 0.3% Triton X-100 (PB-T) for 1 h at room temperature. Subsequently, the slides were incubated overnight at 4 °C with primary antibodies diluted to appropriate concentrations in PB-T. The slides were washed three times in PBS, and incubated for 1 hr at room temperature with fluorescently labeled secondary antibodies. Finally, the labeled sections were washed three times in PBS. Teased nerves were counterstained with diamidino-2-phenylindole (DAPI, Molecular Probes). Images were captured with an all-in-one digital microscope (BZ-X710; Keyence Japan, Osaka, Japan) or confocal microscope (FV100D IX81; Olympus, Tokyo, Japan). Digitized images were transferred to a laboratory computer for analysis using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Statistical analysis.Statistical analysis was performed using Microsoft Excel 2003 (Microsoft Co.). Data were expressed as the mean ± standard deviation (S.D.). A χ2 test was performed for comparisons among fiber types or between CST-deficient and wild-type (Wd-type) mice. A p value of less than 0.001 was regarded as statistically significant because of multiple comparisons.
The amount of DCUN1D2 in adult rat sciatic nerve and brain homogenates was first compared by western blot analysis (Fig. 1A). DCUN1D2 (approximately 30 kDa) was more abundant in the PNS than in the CNS. To identify DCUN1D2 localization in the PNS, we isolated membranous (M) and cytosolic (C) fractions from rat sciatic nerve homogenates. Western blot analysis of these fractions demonstrated that DCUN1D2 was enriched in the cytosolic fraction (Fig. 1A). No difference was observed between the bands recognized by two individual antibodies against the N- and C-terminals of DCUN1D2 (data not shown).

Localization of DCUN1D2 and NEDD8 at myelin paranodes in the rodent sciatic nerve. (A) Western blot analyses of rat brain and sciatic nerve homogenates (left) and fractions of sciatic nerves (right) were performed using an anti-DCUN1D2 antibody. The amount of DCUN1D2 was higher in the rat PNS cytosolic fraction. (B) Teased fibers from 10-week-old mouse sciatic nerves were double-immunostained with anti-DCUN1D2 antibody (green) and either anti-MAG antibody (red, top) as a paranodal marker or anti-pan Na+ channel antibody (red, bottom) as a nodal marker. DCUN1D2 (green) colocalized with MAG (red, top), suggesting that this protein is concentrated at the paranodal myelin. In addition, DCUN1D2 showed some limited immunoreactivity at the nodes of Ranvier where Na+ channels accumulate (red, bottom). (C) Teased fibers from 10-week-old mouse sciatic nerves were immunostained with anti-NEDD8 antibody (green) and either anti-MAG antibody (red, top) or anti-pan Na+ channel antibody (red, bottom). Localization of NEDD8 (green) was similar to that of DCUN1D2. Scale bar = 10 µm.
Immunofluorescence analysis was performed using teased nerve fibers prepared from mouse sciatic nerves to examine the distribution of DCUN1D2. To identify the DCUN1D2-positive regions, two markers were used: MAG to label the myelin paranodes and Na+ channels for the nodes of Ranvier. The pattern of DCUN1D2 immunoreactivity appeared as a pair of clusters along the nerve fibers with a limited region of staining between them (Fig. 1B; green). These clusters colocalized with MAG (Fig. 1B top, red) and were flanked by nodal Na+ channel clusters (Fig. 1B bottom, red), indicating that DCUN1D2 was present at the paranodal myelin where MAG was concentrated. Myelinated Schwann cell bodies were also stained (see Fig. 2A, Fig. 4B).
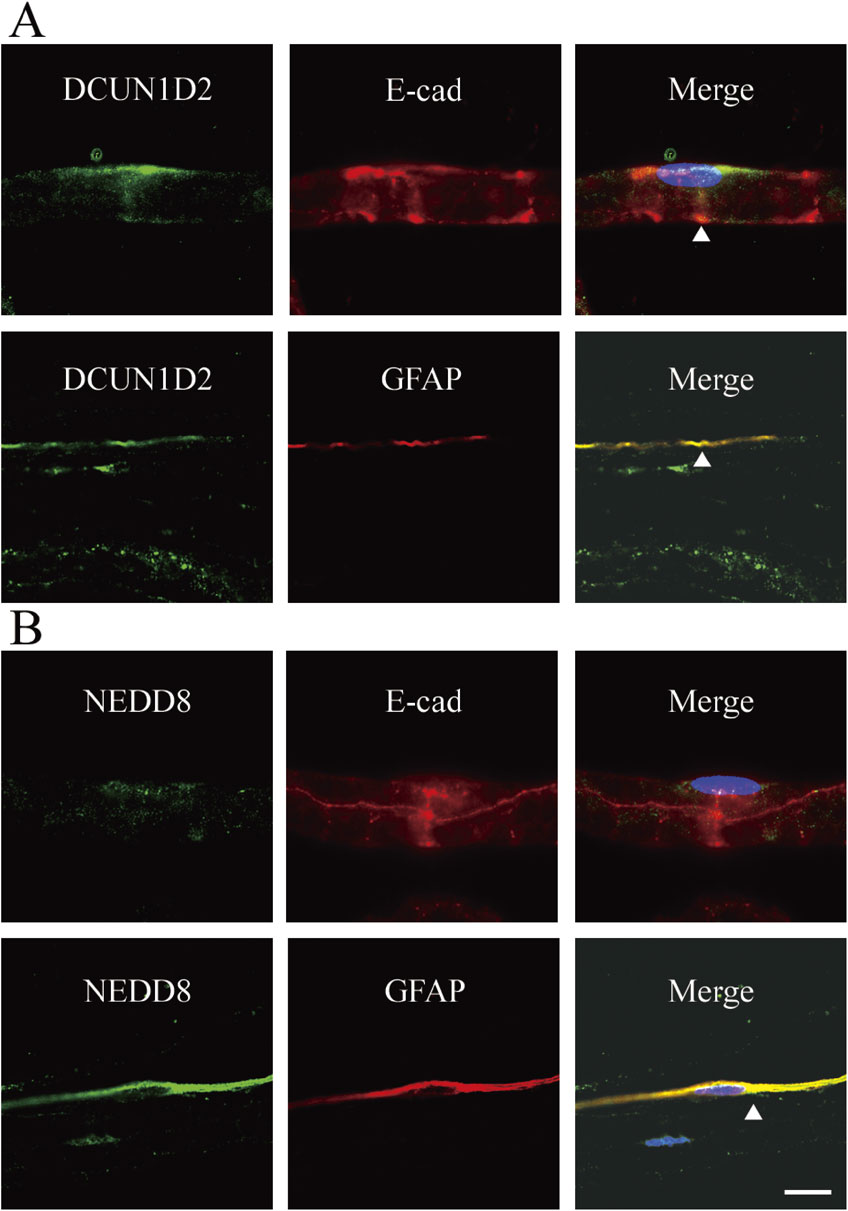
Localization of DCUN1D2 and NEDD8 in Schmidt–Lanterman incisures and nonmyelinating Schwann cells. (A) Teased fibers from 10-week-old mouse sciatic nerves were immunostained with anti-DCUN1D2 antibody (green) and either anti-E-cadherin antibody (red; top) or anti-GFAP antibody (red; bottom). DCUN1D2 partially colocalized with E-cadherin-positive Schmidt–Lanterman incisures (arrowhead; top), although their signals were not completely overlapping. DCUN1D2 was also present in GFAP-positive nonmyelinating Schwann cells (arrowhead; bottom). (B) Teased fibers from 10-week-old mouse sciatic nerves were immunostained with anti-NEDD8 antibody (green) and either anti-E-cadherin antibody (red; top) or anti-GFAP antibody (red; bottom). NEDD8 was not found in E-cadherin-positive Schmidt–Lanterman incisures. In contrast, strong immunoreactivity of NEDD8 was present in nonmyelinating Schwann cells (arrowhead; bottom). Scale bar = 10 µm.
DCUN1D2 is a recently identified member of the DCNL family required for cullin neddylation in vivo.20) Therefore, the localization of NEDD8 in the myelinated fibers was examined by double immunostaining using specific antibodies against NEDD8 and MAG. As shown in Fig. 1C, NEDD8 also colocalized with MAG and was concentrated at the paranodal regions. The staining pattern of NEDD8 was similar to that of DCUN1D2 (Fig. 1B). NEDD8 was also present in Schwann cell bodies similar to DCUN1D2 (data not shown). Thus, the presence of both DCUN1D2 and NEDD8 suggests that the paranodal region may be a site of protein modification by neddylation in the myelin sheath.
DCUN1D2 and NEDD8 are present in nonmyelinating Schwann cells.Schmidt–Lanterman incisures are funnel-shaped small breaks within the compact myelin that contain cytoplasm which connects directly to the cell bodies in myelin of the PNS. In these regions, the autojunctions, including tight, adherence, and gap junctions, connect each layer of myelin. In addition to the paranodal loops, the incisures likely act as connecting channels for molecular transport, including K+ buffering effluxed from the axons. To determine the localization of DCUN1D2 or NEDD8 in Schmidt–Lanterman incisures, double immunofluorescence staining with anti-DCUN1D2 and anti-E-cadherin antibodies was performed (Fig. 2). In addition to the paranodal regions, DCUN1D2-positive signals partially overlapped with the E-cadherin-positive Schmidt–Lanterman incisures. However, no apparent NEDD8 signals were detected in the E-cadherin-positive Schmidt–Lanterman incisures. When GFAP was used as a marker for nonmyelinating Schwann cells,30) DCUN1D2 and NEDD8 signals were found in the GFAP-positive nonmyelinating Schwann cell bodies (Fig. 2).
Accumulation of DCUN1D2 and NEDD8 at the myelin paranodes is influenced by fiber diameter.We next investigated the localization of DCUN1D2 and NEDD8 in different types of nerve fibers. Myelinated fibers were identified by focal clusters of Na+ channels at the nodes of Ranvier (Fig. 3A), and these fibers were classified into three types based on their diameters, designated as Aα- (> 6 µm), Aβ- & Aγ- (3.5–6 µm), and Aδ-fibers (< 3.5 µm). Immunofluorescence staining of teased mouse sciatic nerve fibers showed that DCUN1D2 signal was not found in Aδ-fibers as shown in Fig. 3A. These fibers tended to be located in close proximity to the C-fibers (Fig. 3A), in which Na+ channels were diffusely stained.31) These fibers were often associated with DCUN1D2-positive unmyelinated Schwann cells.

Difference in DCUN1D2 or NEDD8 accumulation patterns at the paranodal regions of Aα-, Aβ & Aγ-, and Aδ-fibers in mouse sciatic nerves. (A) Representative images of double immunostained teased fibers from 10-week-old mice stained with anti-DCUN1D2 (green) and anti-pan Na+ channel (red) antibodies are shown. Myelinated fibers are identified by the presence of nodal Na+ channel clusters as shown in the image. These fibers were classified as Aα- (6 µm<), Aβ & Aγ- (3.5–6 µm), or Aδ-fibers (<3.5 µm) based on the maximum diameter of the internode. DCUN1D2 signal was not detected at paranodal regions in the Aδ-fibers (white arrowheads) that were in close proximity to broadly stained Na+ channel-positive C-fiber bundles. Scale bar = 10 µm. (B) The pattern of DCUN1D2 immunoreactivity in the three nerve types was examined in adult Wd-type sciatic nerves by double labeling as shown in A. DCUN1D2-positive paranodal regions were classified into 3 groups. In the Bilateral group, the nodes were bilaterally flanked by strongly DCUN1D2-positive paranodes. In the Alteration group, the staining pattern of DCUN1D2 was mislocalized from the paranode and diffuse. Representative images of these groups were shown. In the Null group, no apparent DCUN1D2 staining was detected as shown in (A). The total number of counted nodes was: Aδ (n = 241; black columns), Aα- (n = 264; white columns), and Aβ & Aγ (n = 264; grey columns). In most of the Aα-, and Aβ & Aγ-fibers, DCUN1D2 was present in the paranodes. The nodes associated with strongly DCUN1D2-positive paranodes were rarely observed in the Aδ nerves. (C) NEDD8 staining patterns in the three nerve types were also examined. The total number of counted nodes was: Aδ (n = 182), Aα (n = 210) and Aβ & Aγ (n = 449). The color of each column represents the same nerve type as in B. Similar to DCUN1D2, the nodes associated with strongly NEDD8-positive paranodes were rarely observed in the Aδ nerves. Each value represents the SD of the data obtained from three animals. A χ2 test (with 4 degrees of freedom) was performed to determine statistical significance. ※, P < 0.001.
For quantitative analysis, the paranodal regions were classified into three groups according to their DCUN1D2 staining patterns: bilaterally stained paranodes with a pair of clusters (Bilateral group in Fig. 3B, C), diffusely stained paranodes or paranodes with dislocated staining pattern (Alteration group; representative images in Fig. 3B), and no stained paranodes (Null group; shown in Fig. 3A). The number of paranodes from each staining pattern group was counted and percentage was calculated for each fiber type (Fig. 3B). Results showed that the paranodal regions with bilateral DCUN1D2 staining (Bilateral) were not found in Aδ-fibers, and greater than 80% of Aδ-fibers showed no reactivity to DCUN1D2 at the paranodes (Null) (Fig. 3B). In contrast, more than 80% of the paranodes were stained bilaterally, while the DCUN1D2 signal was either dislocated or diffused (Alteration) in the rest of the paranodes of the Aα- and Aβ- & Aγ-fiber types. Only a few paranodes showed no signals for any of these fiber types. NEDD8 staining patterns showed similar results (Fig. 3C). Thus, accumulation of DCUN1D2 and NEDD8 were strongly correlated with the diameter of the myelinated fibers.
Accumulation of DCUN1D2 at the myelin paranodes occurs during the late stages of myelination.Developmental analysis of DCUN1D2 localization was performed by immunofluorescence staining using teased fibers of various ages. Myelinated fibers were identified by the presence of either Na+ channel clusters at the nodes of Ranvier (data not shown) or MAG clusters at the paranodal regions. DCUN1D2 localized to the cell bodies of the Schwann cells at P0 to P21, and staining intensity increased during development (Fig. 4A, B, arrowhead). At P14, although myelinated fibers with Na+ channel clusters were already found, DCUN1D2 was mainly found within the cell bodies and no apparent paranodal staining of DCUN1D2 was observed. By P21, DCUN1D2 clusters at the paranode appeared near the bilateral myelin ends, where they were colocalized with MAG (Fig. 4B, arrow). DCUN1D2 was also found in the perinuclear cytoplasm (Fig. 4B, arrowhead). NEDD8 also appeared at the paranodal regions at P21, which was similar to DCUN1D2 (data not shown). These results indicate that localization of DCUN1D2 and NEDD8 at the paranodal regions occurs during the late stages of the myelination process.

Developmental changes in DCUN1D2 localization in rat sciatic nerves. (A) Double immunostaining of teased fibers from rat sciatic nerves obtained from various developmental ages was performed with anti-DCUN1D2 (green) and anti-MAG (red) antibodies. At P0, P1, P7, and P14, DCUN1D2 was diffusely localized within Schwann cell bodies (arrowheads at top; Schwann cell nuclei were visualized by DAPI in P7 and P14 samples). (B) At P21, DCUN1D2 started to accumulate in the paranodal regions (indicated by arrow). Arrowhead indicates perinuclear staining of DCUN1D2 in the cell body of the same Schwann cell. Scale bar = 10 µm.
Since paranodal axo-glial junctions are formed relatively late during myelination, we examined the influence of these junctions on the distribution of the neddylation-related molecules. Compact myelin appeared normal in CSTKO mice, however, the paranodal axo-glial junctions were disrupted.13) Therefore, the distribution of DCUN1D2 in the CSTKO sciatic nerves was examined (Fig. 5). As shown in representative images in Fig. 5A, the bilateral staining pattern of DCUN1D2 at the paranode was barely detectable even in the large fibers. Quantitative analysis showed a marked reduction of Bilateral stained paranodes and increased Altered or Null paranodes in Aα- and Aβ- & Aγ-fiber types in the CSTKO mice. In Aδ-fibers, the percentage of Null-type paranodes was increased in the CSTKO mice compared to that in Wd-type mice. Similar changes were observed for NEDD8 localization in these fibers (Fig. 6).

Altered localization of DCUN1D2 in CSTKO mice. (A) Representative images of double-immunostained teased fibers from adult CSTKO mice with anti-DCUN1D2 (green) and anti-pan Na+ channel (red) antibodies. DCUN1D2 clusters were either mislocalized (a), diffused (b) or not detected (c) in CSTKO sciatic nerves. Scale bar = 10 µm. (B) The DCUN1D2-positive staining patterns in the three nerve types of adult Wd-type and CSTKO mice were examined. DCUN1D2-positive paranodal regions were classified into 3 groups as described in Fig. 3. The total number of counted nodes was: CSTKO (n = 873; Aα: 264, Aβ & Aγ: 368, Aδ: 241), Wd-type controls (n = 2074; Aα: 301, Aβ & Aγ: 511, Aδ: 389). The percentage of nodes associated with strongly DCUN1D2-positive paranodes (Bilateral) was significantly decreased in all nerve types in the CSTKO mice. Each value represents the SD of the data obtained from three animals. A χ2 test (with 4 degrees of freedom) was performed to determine statistical significance. ※, P < 0.001 vs. control.

Altered localization of NEDD8 in CST-deficient mice. (A) Representative images of double-immunostained teased fibers from adult CSTKO mice with anti-NEDD8 (green) and anti-pan Na+ channel (red) antibodies. NEDD8 clusters were either mislocalized (a), diffused (b) or not detected (c) in CSTKO sciatic nerves. Scale bar = 10 µm. (B) The NEDD8-positive staining patterns in the three nerve types of adult Wd-type and CSTKO mice were examined. NEDD8-positive paranodal regions were classified into 3 groups as described in Fig. 3. The total number of counted nodes was: CSTKO (n = 1445; Aα: 540, Aβ & Aγ: 653, Aδ: 252); Wd-type controls (n = 841; Aα: 210, Aβ & Aγ: 449, Aδ: 182). The percentage of nodes associated with strongly NEDD8-positive paranodes (Bilateral) was significantly decreased in CSTKO mice. Each value represents the SD of the data obtained from three animals. A χ2 test (with 4 degrees of freedom) was performed to determine statistical significance. ※, P < 0.001.
The present study demonstrated that two neddylation-related proteins, DCUN1D2 and NEDD8, are concentrated at the paranodal myelin region as well as in the myelinated and unmyelinated Schwann cell bodies. Accumulation of these proteins at the paranodal myelin was dependent on the diameter of the fibers. Analysis of developing animals and CST-deficient mice suggested that this characteristic distribution was influenced by the presence of paranodal junctions. These results are the first to describe the localization of these proteins within the PNS.
Although NEDD8 was originally found in the embryonic mouse brain,25) neddylation is now believed to have important roles in various cell functions in animals of all ages.17),32) In the brain, neddylation of PSD95 is essential for formation and maintenance of spines in the excitatory synapses,26) although the detailed mechanism is not known. Moreover, dysregulation of neddylation may be involved in pathological conditions including neurodegenerative diseases33),34) and various cancers.35),36) Some substrates of NEDD8 have been identified, but the role of neddylation under physiological conditions is still largely unknown. The most well characterized neddylation substrates are the cullin family members, of which neddylation causes activation of the cullin-RING E3 ubiquitin ligase complex and promotes cullin-based ubiquitination.37) Recently, various non-cullin substrates of NEDD8 have also been reported. This post-translational modification is now believed to be involved in transcription regulation, signaling pathways by receptor tyrosine kinase, apoptosis, DNA damage, and nuclear stress signaling and cell proliferation. In the present study, we showed that DCUN1D2 and NEDD8 were mainly found at the paranodes as well as Schwann cell bodies in the PNS, suggesting that these regions may be the sites for neddylation. The myelin paranode has a unique structure in which autojunctions between each paranodal myelin loop and axo-glial junctions between the axon and myelin are present.8) The paranode contains cytoplasm, and it is thought to be involved in axo-glial interactions as well as molecular transport between the Schwann cell body and deep layer of myelin. Although the presence of neddylation substrates in myelin is still uncertain, molecules concentrated at either the paranode or within the compact myelin are potential candidates. It has been reported that neddylation and deneddylation differentially control transforming growth factor-β type II receptor (TGFβRII) signaling. For example, neddylation of TGFβRII stabilizes the protein and prolongs its signaling.38) In contrast, deneddylation promotes its ubiquitination leading to degradation through lipid raft- and caveolin-mediated endocytosis.38) Epidermal growth factor receptor (EGFR) in the membrane is also regulated by neddylation that causes increased ubiquitination and promotes endocytic internalization.39) Furthermore, neddylation causes membrane targeting of the chemokine receptor CXCR5.40) Thus, it is possible that neddylation may regulate transport of membrane proteins in both directions at the paranode. Identifying the substrates for neddylation in PNS myelin will be the next step in understanding the roles of this modification.
It is important to identify whether myelin ubiquitination is regulated by neddylation through DCUN1D2 since ubiquitination of myelin proteins may be involved in the pathogenesis of several hereditary peripheral neuropathies. In the PNS, 20S proteasome-like immunoreactivity was relatively diffuse but with discernible staining at the paranodal region41) where we demonstrated the presence of neddylation-related molecules. In the trembler-J mouse model for Charcot-Marie-Tooth (CMT) disease type 1A (demyelinating type), aggregates of ubiquitinated PMP22 (peripheral myelin protein 22) and myelin basic proteins (MBPs) have been observed with impaired proteasome activity.41) In addition, a patient with late-onset CMT type 1B caused by a H10P mutation of the myelin protein zero (MPZ) gene demonstrated accumulation of ubiquitinated MPZ inclusions between the myelin and axon, and axonal degeneration without apparent demyelination.42) Thus, ubiquitination processes of myelin proteins are correlated with peripheral neuropathies. Although it is still unknown what types of ubiquitin E3 ligases are involved and whether neddylation of DCUN1D2 is involved in these pathological conditions, it is necessary to examine the role of neddylation in pathological conditions of the PNS.
In the present study, CSTKO mice showed a significant decrease in paranodal staining of DCUN1D2 and NEDD8. In these mice, myelin sulfatide is completely absent and formation of the paranodal axo-glial junctions is severely affected in both the CNS and PNS.11)–13) Since developmental analysis showed that DCUN1D2 and NEDD8 were simultaneously expressed at the paranode during the late stages of myelination, it is possible that mislocalization of these molecules is caused by loss of the paranodal junction. However, the possibility that the absence of sulfatide may induce these changes has not been ruled out. Sulfatide is thought to function in the overall process of NF155 localization to the paranodal regions through a lipid raft.43),44) If DCUN1D2 and NEDD8 are similarly transported by sulfatide, their localization may be affected, as shown in Figs. 5 and 6. Alternatively, it would be interesting to determine if NF155 is a possible substrate of NEDD8 because neddylation/deneddylation processes regulate lipid raft-mediated endocytosis of TGFβRII.38)
During development of the PNS, TGFβRII-mediated signaling controls Schwann cell death and proliferation.45) Our developmental analyses showed that Schwann cell bodies in young animals were strongly immunostained by antibodies against DCUN1D2 and NEDD8. Since neddylation regulates TGFβRII-mediated signaling in blood cells,38) it may be important to know whether this signaling is also controlled by neddylation in Schwann cell development.
The authors thank Dr. Koichi Honke (Kochi Medical School, Nankoku, Japan) for providing the CST-deficient mice.