2016 Volume 92 Issue 4 Pages 109-120
2016 Volume 92 Issue 4 Pages 109-120
This manuscript is dedicated to Prof. Tamio Yamakawa and describes my cooperations on sialic acid-related topics with Japanese scientists during the last 40 years. We studied sialic acids and their O-acetylated derivatives in the sea urchin Pseudocentrotus depressus, in Halocynthia species, and in human and bovine milk. In seafood we mainly searched for N-glycolylneuraminic acid. With synthetic substrates it was shown that sialic acid O-acetylation at C-4 hinders the activity of sialidases, with the exception of viral enzymes. The biosynthesis of Neu5Gc was discussed and the distribution of this sialic acid in dogs followed in modern literature and reviewed regarding their migration. An excellent source of sialic acids is edible bird nest substance (Collocalia mucin) which was used for the synthesis of sialylation inhibitors.
On August 1, 2015, during the 34th Japanese Society of Carbohydrate Research Symposium, Prof. Tamio Yamakawa was honoured by a special session, not only because he had received the “Lifetime Achievements in Sialoglycoscience Award 2014” by the International Sialic Acid Community, introduced by Mark von Itzstein, Griffith University, Australia, but also because he was named “Person of Cultural Merit” (Bunka Kôrôsha, 文化功労者) by the Ministry of Education, Culture, Sports, Science and Technology (Mombu-Kagaku-shō). Since I could not deliver my laudatory speech in person I am following a long-standing invitation to contribute to this journal and report on very rewarding cooperations with Japanese colleagues over a period of 40 years. I am very thankful that I and my associates had the opportunity to cooperate with these colleagues on different topics, but always centered around sialic acids, especially after the First International Sialic Acid Meeting, Tokyo (1985), organized by Haruo Ogura and Tamio Yamakawa, and the Japanese-German Sialic Acid Meeting, Berlin (1988), organized by T. Yamakawa, Akemi Suzuki and myself. All work was done in harmony and I am convinced that my wife Elfriede and I learned to better understand the Japanese scientific and social life. We travelled to Japan whenever possible, attended many meetings, gave lectures and took pleasure in Japan’s exciting natural beauty and cultural traditions.
T. Yamakawa had spent a sabbatical in Ernst Klenk’s laboratory in Cologne working on gangliosides. In Japan, he became the father of glycolipids and with his pioneering work he developed glycobiology not only in his home country but also throughout the world.1),2)
Sialic acids (Sia) are a blooming and challenging area of research, followed by many laboratories around the world. These monosaccharides are very diverse in their structure and are constituents of many oligosaccharides, glycoproteins and glycolipids, especially of cell membranes and of secreted products, e.g. the mucins and milk oligosaccharides. They are wide-spread, occur both in pro- and eukaryotes, in particular in bacteria and are found in some viruses, protozoa, a few insect species and regularly in deuterostome animals, from echinoderms onward. Their chemistry varies; the Sia family, representing about 50 members, are all derivatives of the 5-amino-2-keto-3-deoxy nononic acid, the “neuraminic acid” (Fig. 1). The latter is acylated at the amino group with an acetyl or glycolyl group, respectively. The various hydroxyl residues are esterified with acetic, phosphoric, sulfuric or lactic acid or methylated.2)–4) The most frequent Sias are N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc) and O-acetylated derivatives thereof (most frequently N-acetyl-9-O-acetylneuraminic acid, Neu5,9Ac2) and N-acetyl-9-phosphorylneuraminic acid, although the latter is only a short-lived metabolic intermediate. A deaminated derivative of neuraminic acid is 2-keto-3-deoxy nononic acid (Kdn) which was found in glycans from animals and microorganisms.3),4) This substance was discovered in 1968 by the late Yasuo Inoue and his colleagues.5) Legionaminic acids, being modified at the glycerol side chain, of some bacteria species can also be considered as members of this sugar family. These acidic amino sugar molecules all belong to the growing family of nonulosonic acids (NulOs).
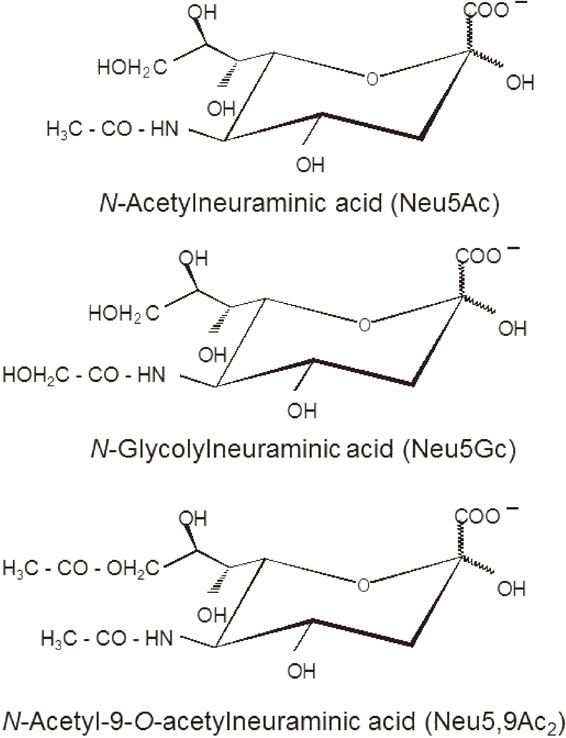
Structures of the three most frequently occurring sialic acids N-acetylneuraminic acid, N-glycolylneuraminic acid, and N-acetyl-9-O-acetylneuraminic acid. Neu5Ac is the mother molecule of the other Sia. O-Acetyl residues occur at C-4, C-7, C-8, and C-9 of Neu5Ac and Neu5Gc. The 9-hydroxyl can be lactylated or phosphorylated and at C-8 a sulfate or a methyl group can be found in some biological materials.3),4)
The occurrence of the different Sia is genetically regulated and depends on the animal species and the kind of tissue where it is expressed. During ontogenesis, ageing, change of cell functions, or malignant differentiation their quality and quantity may change. Sia is an essential molecule of life in each cell from animals which possess the genetic programme of Sia biosynthesis, which is regularly the case in animals from echinoderms to mammals in evolution. It exerts a multitude of functions in practically all aspects of cell biology, such as fertilization, growth, ageing, molecular and cellular interactions, ion transport, cell signaling, neuronal functions, immunological processes, malignant growth and the biology of infections.3),4) Presently, the eminent role in nutrition and bacterial colonization in intestine is recognized and studied in detail (see below).
Main research is now focussed on these biological and pathological aspects in various systems. Our basic knowledge on the occurrence and complicated structure of neuraminic acid itself and the various sialic acid forms and the numerous metabolic reactions and genes involved in the synthesis, modification, transfer, and breakdown, and principal (patho-physiological) functions of Sia was acquired since the 1940s.3) At that time, Sia research was still in its infancy; after a long time and many efforts the structure and basic metabolism of Sia was elucidated and first insights into the biological role were obtained.
In 1967, I started my work on sialic acids in Hans Faillard’s laboratory at the university of Bochum, whose mentor had been Ernst Klenk in Cologne. Sia was isolated and separated from bovine submandibular glands, liberated from the mucin by mild acid or sialidase, on cellulose powder in a butanol-propanol-water phase.6) This method was in use by Masakichi Motomiya from Sendai in the laboratory of Ernst Klenk and Hans Faillard in Cologne and had been developed on the basis of Sia separation on thin layer chromatography.7) This separation method was very efficient and allowed the isolation of a large number of mainly O-acetylated Neu5Ac and Neu5Gc derivatives in pure form and good quantities. With these molecules we established the first mass spectrometric analysis (GC-MS) of Sia, allowing for instance the localization of the labile O-acetyl groups on Sia.8) As standard for analyzing 4-O-acetylated sialic acids in equine submandibular glands we used 4-O-acetylated Neu5Gc, which was isolated from the hematoside of horse erythrocytes, discovered by Yamakawa in 1951.9) With this work he had been the first to show that glycolipids are located in the cell membrane and that Sia is exposed on the cell membrane. The biological activity of erythrocyte glycolipids was also demonstrated by Yamakawa for the first time.
Since Sia analysis was in the centre of my interest at the beginning of my work in this field, I shall first describe some analytical projects with Japanese colleagues. It was in 1980 that in cooperation with Kyoko Hotta we published the occurrence of large amounts of Neu5,9Ac2 in a sulfated sialoglycoprotein from the jelly coat of eggs from the sea urchin species Pseudocentrotus depressus (Okayama).10) Here we used the GC-MS method newly developed by Hans Kamerling and Hans Vliegenthart in Utrecht (The Netherlands).8) These analyses were planned when I attended the 8th International Symposium on Carbohydrate Chemistry in Kyoto in August 1976, where I spoke on “Properties of N-acetylneuraminate monooxigenase”. At that time it had become known that sialic acids in various forms and linkages occur in echinoderms and in those animals which developed later in evolution, finally leading to the mammals.3),4) We therefore also looked in Ascidiaceae, belonging to the phylum urochordata resp. tunicates like Halocynthia roretzi, H. aurantium (Fig. 2) and H. hilgendorfi for the possible occurrence of Sia. These animals are invertebrates but close to the vertebrates. The Halocynthia species were provided by Kazuhiko Konishi from the Tohoku University at Sendai. We investigated the body wall, liver, gonads, gills and muscle, but did not find Sia after mild acid hydrolysis and thin-layer chromatography of the samples. Only in the ganglia a sialic acid-positive reaction was obtained by colorimetry. Even an extremely sensitive method for study of the biosynthesis of Sia by these animals, the incubation of living tissue slices (with the exception of ganglia due to the lack of tissue), or of frozen, homogenized tissues with radioactive N-acetylmannosamine, under conditions which lead to Sia formation in vertebrate tissues, did not result in the formation of radioactive Neu5Ac. It thus can be assumed that in Halocynthia the role of Sia is carried out by other acidic sugars like uronic acids which frequently are found in lower animals. The recent report of the isolation of a glucuronic acid containing glycosphingolipid from H. roretzi11) points to this direction. Of course, more Ascidian (sea squirt) species and animals from related groups may be investigated for the unequivocal (non-)occurrence of Sia.
Halocynthia aurantium (left) and H. roretzi (right). Courtesy of Prof. Kazuhiko Konishi.
With Toshiaki Suguri, sent by Snow Brand Milk Products Co., Ltd., Tokyo, we studied, starting in 1987 and keeping in touch for almost 20 years, sialic acids in human milk. During the last 100 years researchers became aware and interested in the chemistry and biology of oligosaccharides from the milk of mammals, especially man. Observations had shown that human newborns grew and survived best when breast-fed. Jean Montreuil, Lille (France) reported the meager results of nuns who nourished newborn babies, deposed at the door of a monastery, with cow milk due to the lack of human milk. The babies’ survival rate was very limited. Today it is known that bovine milk contains different and less oligosaccharides and much less sialic acids than human milk.12)–15) In human milk more than 130 and often sialylated oligosaccharides, glycoproteins and glycolipids were analyzed, and their effects on bacterial colonization of the intestine, immune reactions and protection from infections are well established.16),17) Montreuil and Mullet observed in 1960 that milk of a woman had highest Sia content (1400 mg/l) at the first day of lactation which rapidly declined during the following days.18) Several investigators showed a several fold decline of sialic acid concentration in oligosaccharides, glycoproteins and sialyllactose of human milk during the first 10 to 20 weeks of gestation.19) This was also observed in other mammals like the cow, goat or ewe.20) The high amount of Sia secretion in milk shortly after child-birth may mirror the high rate of ganglioside biosynthesis of the brain of human and rat newborns and milk Sia is a supplementary source of glycoconjugate-bound brain Sia.21) Both rat and human show two periods of an increase of the ganglioside concentration in the brain, at birth and in man also in the third intrauterine trimester.22) Feeding of Sia to newborn rats, consequently, leads to a significant increase of the Sia content in brain and improved learning abilities of the rat.23)–25) The same was observed in pigs, which concomitantly gained in intelligent performance.26) Besides nutritional aspects, Sia may have also an important role on non-immunological protection of infants from a wide range of pathogens and toxins.
Since Sia are often O-acetylated, we focussed on such modified Sia in human milk. Milk samples from various stages of lactation were collected from 2,434 healthy Japanese mothers, pooled, and the Sia liberated by mild acid hydrolysis and analyzed by fluorimetric HPLC.3) The results are shown in Figs. 3 and 4. The amounts of Neu5Ac and Neu5,9Ac2 decreased from 3.8 mg/ml to 0.9 mg/ml and from 0.04 mg/ml to 0.01 mg/ml, respectively, in the course of about 100 days. The ratio of Neu5Ac to Neu5,9Ac2 remained constant (1:100) during this lactation period (Suguri & Schauer, unpublished).

Course of the concentration of N-acetylneuraminic acid (right) and N-acetyl-9-O-acetylneuraminic acid (left) in human milk during lactation.

Fluorimetric HPLC analysis of sialic acids in a sample of human milk.3) A: Run of acid-liberated free Sia. B: After aldolase (sialate-pyruvate lyase) treatment of this sample, which splits Sia into pyruvate and acylmannosamine, the Sia peaks were much smaller, showing the authenticity of these Sia peaks.
A similar trend of Sia during lactation was observed in cow milk (Fig. 5). However, the decline was much faster than in human milk. The amounts of Neu5Ac and Neu5,9Ac2 decreased from 2.6 mg/ml to 0.3 mg/ml and from 0.28 mg/ml to 0.02 mg/ml, respectively. Most of this decline had already occurred in the first 30 h after delivery. The ratios of Neu5Ac to Neu5,9Ac2 decreased during lactation from 9.7% to 5.5%. This also shows an about 10-fold higher O-acetylation grade of Sia in early cow milk than in man (unpublished). Western Blot analysis of human milk proteins also revealed several protein bands containing 9-O-acetylated Sia. These were detected by an overlay technique using influenza C viruses which bind to Neu5,9Ac2.
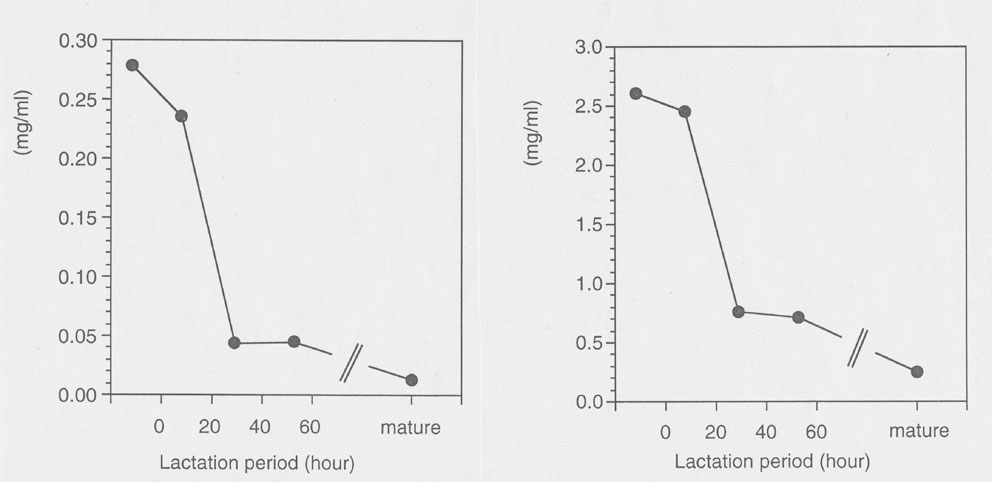
Course of the concentration of N-acetylneuraminic acid (right) and N-acetyl-9-O-acetylneuraminic acid (left) in bovine milk during lactation.
The function of O-acetylated Sia in milk has not been studied experimentally, however, an anti-infection role can be assumed, since various viruses (e.g. influenza C and corona viruses) bind to O-acetylated Sia and in this way are absorbed.27) O-Acetylated Sia are relatively stable in intestine, since the action of intestinal sialidases is much reduced by Sia O-acetylation. It can be assumed that bacterial colonization of intestinal mucosa is regulated by Sia esterification. A similar role is attributed to oral saliva, the Sia of which are also partly O-acetylated.3) This modification is extremely pronounced in bovine saliva, which is highly O-acetylated and composed of various Sia types: Is this striking phenomenon involved in the microbial colonization of the cow’s rumen? More research on the immunological effect of milk and saliva sialic acids and oligosaccharides seems to be necessary. Unsubstituted Sia should be easily hydrolyzed from its glycosidic linkage, absorbed from the intestine (as Sia or its cleavage product N-acetylmannosamine)28) and used for tissue maturation, especially the brain, and this may explain why human milk is less O-acetylated.
During the cooperation with T. Suguri and Torkel Wadström from Lund University (Sweden) I remember discussions and successful efforts to inhibit the binding of Helicobacter pylori to stomach epithelia by sialylated milk proteins.
With Yasuro Shinohara and his group from the Laboratory of Medical and Functional Glycomics, Hokkaido University, Sapporo, we are investigating the glycans and especially Sia of sea foods, i.e., of several fish species as well as shrimp, crab and sea urchin.29) In shrimp and crab no Sia was found. One focus is directed on the occurrence of Neu5Gc in sea food, since this Sia is a xeno-antigen and can be absorbed from the intestine in small quantities and incorporated into tissue glycoconjugates.28) There it exerts antigenic reactions, which may lead to inflammation and eventually to cancer, e.g. of the colon.30) Especially red meat (from cow, pig, goat, lamb) has a high Neu5Gc content, in contrast to birds, like chicken.31) The glycomes of N- and O-glycans from glycoproteins and glycosphingolipids from meat and roe of eight popular sea foods were analyzed with a recently established analytical technique. Many different oligosaccharides were detected. Sia were abundant in fish and sea urchin glycans but absent in shrimp and crab. (In the glycolipids of the latter uronic acids were found). Sialylated glycans were more frequent in fish roe than in meat. Neu5Gc was present in all fish species but in very low quantities (most around 1% of the Sia fraction). Only in salmon roe O-glycans high levels of this Sia were found. O-Acetylated Sia were also often detected. From these studies it can be concluded that most parts of sea foods are safe with regard to the xeno-antigen Neu5Gc.
Two projects on the metabolism of Sia focussed on sialidase and Neu5Ac hydroxylase. With sialidases we were mainly interested in their substrate specificities, especially with differently O-acetylated Sia. This was also of practical importance, enabling the localization of O-acetyl groups in glycan-bound Sia. Whereas the glycosidic linkage of unsubstituted Neu5Ac or Neu5Gc and their 7- or 9-O-acetylated derivatives are hydrolyzed by all sialidases, although at different rates, 4-O-acetylated Sia is resistant towards mammalian and bacterial enzymes. Viral sialidases can slowly act on this Sia. The 4-O-acetylated Sia in horse hematoside isolated by Yamakawa or in equine submandibular gland mucin served as standard in these studies. Kimio Furuhata, member of the group of Haruo Ogura, Kitasato University, synthesized 4-methylumbelliferyl (MU)-α-glycosides of Neu4,5Ac2, Neu5,7Ac2, and Neu5,9Ac2. Vibrio cholerae and Clostridium perfringens sialidase could not hydrolyze MU-Neu4,5Ac2, only the other two O-acetylated species, although at slower rate than MU-Neu5Ac.32) In contrast to mammalian and bacterial sialidases, viral sialidase, as tested with the fowl plague virus enzyme, was able to slowly release Neu4,5Ac2, at about 5% the rate of Neu5Ac. This difference is due to a pocket in the activity centre of the viral sialidase, allowing access of 4-O-acetylated Sia. Relenza, 4-guanidino-2-deoxy-2,3-di-dehydro-Neu5Ac (4-guanidino-Neu5Ac2en), perfectly fits into this pocket and thus represents a potent inhibitor of influenza virus sialidase and spreading of the viruses, respectively.33)
N-Glycolylneuraminic acid (Neu5Gc) was the second topic, mainly discussed with Yamakawa and A. Suzuki. As mentioned before, Yamakawa, in 1951, had isolated hematoside (sialosylgalactosylglucosyl-ceramide) from horse erythrocyte membranes which contains mainly (4-O-acetylated) Neu5Gc.9) Due to this striking finding together with the occurrence of Neu5Gc in many other biological materials including tumors, studies on the biosynthesis of this Sia type began in the 1970s. It turned out that a monooxygenase (hydroxylase) converted Neu5Ac to Neu5Gc with NAD(P)H as cofactor,34) but it took 18 years more to show that CMP-Neu5Ac is the natural substrate for this hydroxylase as studied in pig submandibular gland.35) The group of A. Suzuki elucidated the complex mechanism of electron transport leading to the conversion of Neu5Ac to Neu5Gc, in which cytochrome b536) and cytochrome b5 reductase37) are involved. In 1995 they described the cloning of mouse liver CMP-Neu5Ac hydroxylase.38) The same group also was the first to observe that the hydroxylase gene (CMAH) was defect in humans thus explaining that normally Neu5Gc is absent in man.39) The Rieske sulfur-iron center, which is involved in the hydroxylase reaction, is missing due to an Alu-mediated inactivation of the CMP-Neu5Ac hydroxylase gene.40) Very small amounts of Neu5Gc found in human tissues (around 0.1% of the Sia fraction) are assumed to be of nutritional origin.41)
Another topic, first studied by Yamakawa’s group, which attracted our interest, since we also investigated the occurrence of Neu5Gc in a variety of animals, was the observation that dogs can be divided into two groups, one expressing Neu5Gc and the other only Neu5Ac. As studied with the hematoside from erythrocytes of dogs from Northern China, Korea and the Southern part of Japan (e.g. Shiba dog) expressed Neu5Gc besides of Neu5Ac, in contrast to the Hokkaido dog from the North of Japan and to European dogs which revealed only Neu5Ac.42),43) From pedigrees of some dog families, inheritance of dogs with Neu5Gc-hematoside was found to be dominant. These observations are interpreted that dogs migrated from China via Korea to Japan (Fig. 6).44) Parker et al. (2004) assume that these Asian breeds diverged from the common Western dog breed thousands of years ago.45) The Hokkaido dog in the North of Japan, however, expresses only Neu5Ac and is therefore believed to be derived from European dogs, which migrated to the East on the Siberian route (Fig. 6). Interestingly, The Australian dingo, which is thought to be the ancestor of modern domestic dogs (Canis familiaris) also expresses only Neu5Ac.44) The same is the case with the jackals, which live between India and North Africa (Fig. 6). It would also be interesting to study the American coyotes, and, of course, wolves.
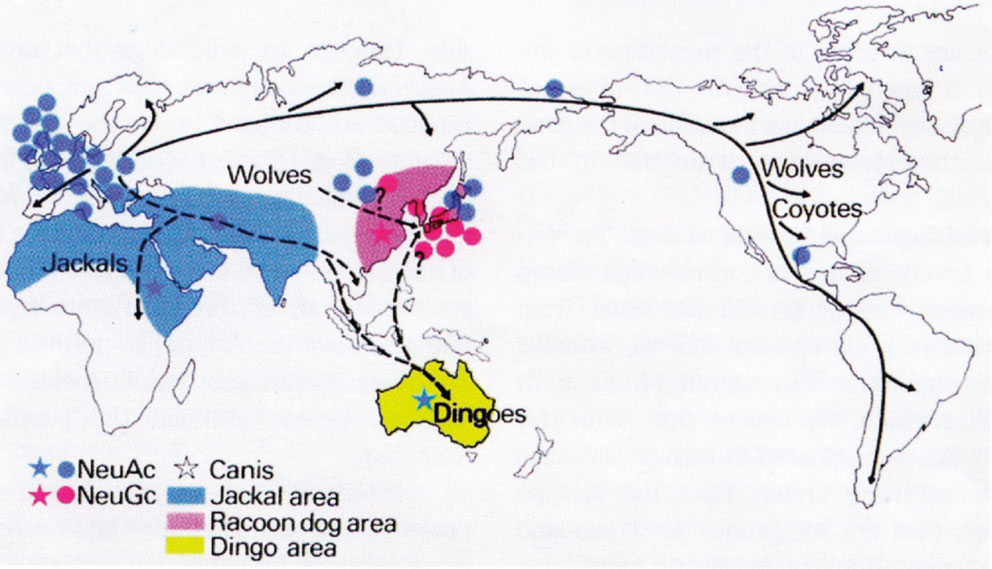
Geographical distribution of dogs and related animals and their migration indicating the possible origin of Japanese dogs (red).44) The figure is reused from the proceedings of the Japan Academy, Ser. B, with permission of the publishers.
European dogs lack Neu5Gc not only in erythrocytes but also in other cells or fluids. In submaxillary gland mucin only Neu5Ac was found46) as well as in milk oligosaccharides of different dogs47) or the beagle dog.48) In a study on dystrophin-deficient dogs (Golden retriever) maximum 2% Neu5Gc was found in muscle tissue.49) In a study on the binding of canine parvoviruses to Neu5Gc Löfling et al.50) found that transcripts of CMAH, which generates Neu5Gc from Neu5Ac, are at very low levels in Western dog breed cells. A. Suzuki assumes that in Western dogs a mutation in a gene related to transcriptional regulation of Neu5Gc expression occurred (personal communication). Recent BLAST analysis has shown that the CMAH gene in Western dogs’ genome, studied with the Boxer species, seems to be present (V.S. Gnanapragassam, personal communication). However, more studies are necessary. Both dogs and humans have a high incidence of tumors and often lack Neu5Gc and eat red meat, they also could produce anti-Neu5Gc-antibodies which may contribute to inflammatory and malignant diseases.30)
The last 50 years revealed that Sia is involved in many cell-biological and pathological processes and that it has a dual functional role.51),52) It can be a recognition signal, which interacts with receptors on mammalian cells, e.g. the Siglecs, or on viruses, best studied with influenza viruses. On the other hand, Sia can function as a mask, covering recognition sites on the surface of proteins or preventing subterminal galactose residues of glycan chains from being recognized. One of the most important observations in glycobiology is due to Morell, Ashwell et al.,53) who discovered that desialylated serum glycoproteins bind to hepatocytes followed by endocytosis and degradation. After (partial) desialylation, blood cells like erythrocytes, lymphocytes and thrombocytes experience a similar fate and bind to liver Kupffer cells and peritoneal macrophages.54),55) Erythrocytes and a part of the thrombocytes are phagocytosed and degraded (Fig. 7). This process is mediated by a galactose-recognizing receptor, isolated from rat peritoneal macrophages, which can be inhibited best by clustered galactosides.56)–58)
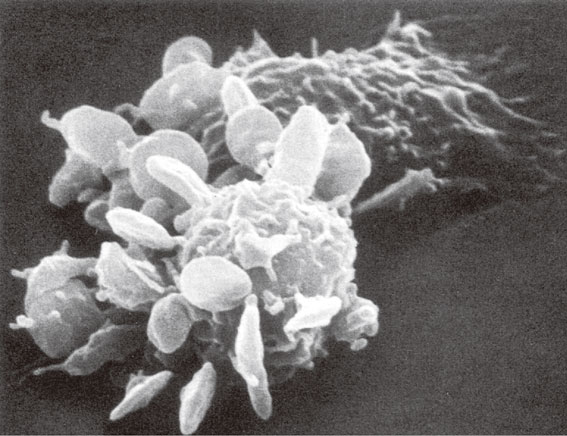
Scanning electron microscopy of sialidase-treated rat thrombocytes bound to homologous peritoneal macrophages. The breadth of the picture corresponds to 15 µm.
In cooperation with Teruo Yoshino from the International Christian University, Tokyo, we studied which residues at the galactose molecule are most important for the interaction with the lectin. For this purpose Yoshino substituted various hydroxyl groups of galactose in lactose with O-methyl groups. It turned out that the hydroxyl group at C-4 is most important for the recognition of galactose by the receptor.52),59),60) Methylation of the hydroxyl at C-3 had some, but much weaker inhibitory effect on receptor binding. Methylation of C-2 and C-6 had no effect. Epimerization at C-4, leading to glucose, and introducing a Cl-atom at C-4 also abolished binding. Thus, the macrophage galactose receptor interacts with the hydrophilic A-side of galactose.
Two other projects in which I was involved were with Chugai Pharmaceutical Co., Ltd., Tokyo, and with MECT Corporation, formerly Kantoishi Pharmaceutical Co., Ltd., Tokyo. At the International Carbohydrate Symposium in Kyoto in 1976 I had met Takashi Okuyama and we started discussions on mammalian sialidases, which were continued on an advisory basis and concentrated mainly on the glycosylation and biological activity of erythropoietin (EPO). This hormone was first expressed in bacteria, but did not survive in the blood stream because it was not glycosylated. Only after the expression in mammalian cells an EPO with biological activity was obtained, but only with low and rather variable performance. The reason was an unstable sialylation grade with unoccupied terminal galactose residues of the carbohydrate chains of EPO, by which the protein could be captured by the galactose receptor of liver and macrophages (see above). Only after taking care of full sialylation of the glycan residues a highly active hormone with good and constant pharmacokinetic properties was obtained. The advisory discussions of the development of an optimally active EPO in the 1980s was a great scientific experience for me.
Kantoishi Pharmaceutical Co., Tokyo, contacted us in 1981 regarding the availability of synthetic Neu5Ac, which also Haruo Ogura used for synthetic purposes. Interest in this monosaccharide grew worldwide at that time, since only few sources for analytical and synthetic purposes were available. The price for free Neu5Ac on the market was high, as the synthetic procedures yielded only relatively low amounts and most natural sources contained mixtures of Sia. For some while, before he passed away, a sialuria patient in France excreted large amounts (around 10 g/day) of free Neu5Ac in urine, which could easily be purified.61) After Kathan and Weeks had published in 196962) the isolation of Sia from Collocalia mucoid (edible bird nest substance), from the Chinese swiftlet (Genus Aerodramus collocalia), another source for the isolation of large quantities of Neu5Ac was found. Wieruszeski and coworkers described the structure of the monosialyl oligosaccharide from the salivary gland mucin of this swiftlet.63) When I worked at the University of Bochum it was difficult to find a supplier, therefore I analyzed samples of swallow nest soup from a Chinese restaurant and found one which contained high Sia concentrations. Finally I succeeded in finding a company in Germany to sell this substance, which enabled the isolation of larger quantities of pure Neu5Ac. The European house martins (Delichon urbicum) produce only low amounts of Neu5Ac (personal studies).
From Ogura and his book64) we learned about the health benefits of the expensive Collocalia mucin, which has been in use in China for hundreds of years. For example, it is a potent binder of influenza viruses and may regulate the adhesion of microorganisms in the oro-gastro-intestinal tract. We also had the opportunity to sample various dishes prepared with this substance by a Chinese chef.
Based on the experience we had gained in isolation of Neu5Ac from Collocalia mucoid I could advise MECT Company correspondingly. This led to a long cooperation with various colleagues, especially Yoshiyasu Shitori and Kinji Takada. The Neu5Ac was sold as free monosaccharide, used among others for the synthesis of, for instance, sialylation inhibitors or the preparation of pharmaceuticals, e.g. expectorants for patients with various bronchial diseases and anti-influenza drugs. CMP-Neu5Ac was also a valuable product. An analogue of this substance, 5-fluoro-2′,3′-O-isopropylidene-5′-O-(methyl 5-acetamido-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonate)-uridine (KI-8110, structure depicted in)65) inhibited sialylation of metastatic cell lines and reduced the formation of metastases when the cells were applied to nude mice.66),67) Harvey and Thomas68) and Schmelter and Ivanov in my laboratory showed that KI-8110 does not achieve this effect via a modulation of sialyltransferase activity but by inhibition of the transport of CMP-sialic acid into Golgi vesicles (unpublished).
With a similar substance, KI-8115, having a modified adenosine residue bound to acetylated and carboxyl-methylated Neu5Ac, instead of uridine, synthesized by Ogura et al.,68) we could show in 1992 that the propagation of influenza viruses in cell culture was significantly inhibited (Odenthal-Schnittler, M., Schwarz, R.T. & Schauer, R., unpublished). Further, Neu5Ac nucleoside derivatives, the inosine, uridine and cytosine glycosides were synthesized according to the method of Ogura.69) However, they had no significant inhibitory effect on the α2–3 and α2–6 sialyltransferases tested. Good inhibitors of these enzymes are cytidine and, much better, its nucleotides 5′-CMP and 5′-CDP.65) KI-8110 has also no effect on CMP-Neu5Ac synthase.
In the last years, the search for efficient sialyltransferase inhibitors was revived. A good inhibitor is peracetylated 3Fax-Neu5Ac, which is converted in the cell into CMP-3Fax-Neu5Ac, inhibiting sialyltransferases,70) and it is hoped that such substances can be successfully used for the reduction of sialylation of cancer cells.

Roland Schauer was born in 1936 in Stuttgart-Bad Cannstatt, Germany. After studying medicine at the University in Tübingen (1955–1961) he continued his scientific training by studying biochemistry at the same university (1962–1966). There he came into contact with Alfred Gottschalk, who had cooperated with Ernst Klenk, Cologne, the place where Tamio Yamakawa had done some research. Upon advice of Hans-Dieter Klenk, R. Schauer applied for a position with Hans Faillard and started his work on sialic acids at the newly founded Ruhr-University of Bochum in spring 1967. After his habilitation in 1970 and working as an Associate Professor at the same university he followed a call as Full Professor and Director of the Institute of Biochemistry at Christian-Albrechts-University at Kiel in December 1976, where he is staying on from 2001 as Emeritus Professor. The varied aspects of sialic acids keep him fascinated and active.
In addition to those colleagues with whom I cooperated directly I want to thank all those scientists who also became friends and who are too numerous to be listed here. Thinking about sialic acid is thinking of Japan and her scientists, and I am eager to learn more about this field of research which still poses a lot of questions.