2017 年 93 巻 10 号 p. 832-840
2017 年 93 巻 10 号 p. 832-840
Volvocine algae constitute a green algal lineage comprising unicellular Chlamydomonas, four-celled Tetrabaena, eight to 32-celled Gonium, and others up to Volvox spp., which consist of up to 50,000 cells. These algae proliferate by multiple fissions with cellular growth up to several fold in size and subsequent successive cell divisions. Chlamydomonas reinhardtii cells produce two to 32 daughter cells by one to five divisions, depending on cellular growth in the G1 phase. By contrast, in this study, we found that Tetrabaena socialis and Gonium pectorale cells mostly produced four and eight daughter cells by two and three successive divisions, respectively. In contrast to C. reinhardtii, which is committed to cell division when the cell has grown two-fold, T. socialis and G. pectorale are committed only when the cells have grown four- and eight-fold, respectively. Thus, our results suggest that evolutionary changes in cellular size for commitment largely contributes to the emergence and evolution of multicellularity in volvocine algae.
Many eukaryotic algae belonging to diverse lineages proliferate by multiple fission events (also called palintomy), in which cells grow to many fold of their original size and then undergo several rounds of division without intervening growth.1),2) For example, a single cell of Chlorella spp. forms two, four, eight, 16, or 32 daughter cells in the mother cell by one to five rounds of successive cell division, and the daughter cells then hatch out of the mother cell. In some cases, a defined number of multiple fission events result in a coenobium (a colony containing a fixed number of cells), as seen in Scenedesmus spp. and Pediastrum spp., and further results in more complex multicellularity, as observed in volvocine algae.3)
Volvocine algae are a green algal lineage, which include the unicellular genera Chlamydomonas and Vitreochlamys extending to ranges of size and levels of complexity from unicellular to complex multicellular organisms with cellular differentiation.4) The smallest multicellular forms consist of four Chlamydomonas-like cells that are held by an extracellular matrix as in Basichlamys and Tetrabaena of the Tetrabaenaceae family.4),5) The Goniaceae includes Gonium, which range from eight to 32 cells, and Astrephomene, which consist of 32–64 cells.4) The diverse Volvocaceae include several genera varying from 16 cells up to 50,000 cells and, in the genus Volvox, cell differentiation systems evolve and reproductive cells differentiate from somatic cells.
Cell cycle progression in eukaryotic algae has been well studied in Chlamydomonas reinhardtii, a unicellular volvocine species. Like many other eukaryotic algae, the cells grow during a prolonged G1 phase that lasts between 10 and 14 h, during which cells enlarge by more than two-fold in size.6),7) Under favorable conditions, the cells grow in volume by more than 10-fold during the G1 phase.7),8) At the end of G1 phase, cells undergo successive rounds of rapidly alternating S and M phases to produce two, four, eight, 16, or 32 daughter cells depending on size of the mother cell.7),9)–11) Daughter cells then hatch out of the mother cell to begin the cycle again. In a typical diurnal cycle (e.g., 12 h of light/12 h of dark), the cell cycle becomes synchronized such that growth occurs during the light phase and cell division (S/M phases) occurs in the dark.7),11),12) The separation of G1 and S/M phases into light and dark conditions was suggested as an adaptation of motile green algae to flagellation constraint, a situation in which cells must resorb their flagella prior to cell division in order to use their basal bodies to coordinate chromosome segregation and cytokinesis.7),13) During light periods, flagella-dependent phototaxis is required to optimize light absorption for photosynthesis, and hence cell division is delayed to the dark period as phototaxis is not then required.7)
The multiple fission events of C. reinhardtii are controlled by two mechanisms involving a commitment point and size control in the cell cycle. In addition, cell cycle progression is further regulated by circadian rhythms so that cells undergo S/M phases only during (subjective) night. The commitment point is in the late G1 phase for C. reinhardtii, corresponding to the “start” in yeasts or the “restriction point” in mammalian cells, and it is a point at which cells can complete at least one round of cell division even when nutrients and/or light for further cellular growth are withdrawn.10),12),14) C. reinhardtii mother cells need to grow at least two-fold to pass the commitment point and divide at least into two daughter cells.7),8) Recent studies suggested that the retinoblastoma (RB)-E2F-DP pathway is involved in the commitment and G1/S transition in C. reinhardtii.12),15),16) However, reaching the commitment size is not sufficient for the cell to enter the S/M phase, and entering the S/M phase has to undergo further cellular growth until the (subjective) evening so that cell division occurs only during night. Once the cells enter the S/M phase, the number of S/M cycles that each mother cell undergoes is governed by cell size: large mother cells divide more times than small mother cells, so that daughters of a relatively uniform size distribution are always produced.9),10) In addition, a recent study suggested that a special type of cyclin-dependent kinase, CDKG1, functions as a sizer in C. reinhardtii and was suggested to act through the RB pathway.8)
The regulations observed in C. reinhardtii were suggested to be conserved among volvocine green algae.17)–19) For instance, Tetrabaena and Gonium also undergo multiple fission events to form daughter colonies by keeping cells attached after multiple fissions. Each cell of a Tetrabaena socialis colony acts as a mother cell and undergoes two successive cell divisions that subsequently produce a four-celled colony and hatches out from the mother cell. In a similar manner, each Gonium pectorale cell undergoes three or four cell divisions producing an eight- or 16-celled colony. Thus, it was suggested that cell cycle regulation and cell–cell adhesion have been modified to promote multicellularity. Based on this assumption, T. socialis and G. pectorale cells must complete at least two and three cell divisions, respectively, before hatching out from the mother cell, in contrast to unicellular C. reinhardtii cells which can hatch out even after only one division has been completed. However, it has been unclear how the minimum cell division number is defined and regulated to maintain multicellularity.
As a first step to address this issue, we characterized how T. socialis and G. pectorale cells are become committed to cell division. Here, we show that T. socialis and G. pectorale are committed to enter S/M phase only when the cell has grown beyond four-fold and eight-fold of the daughter cell size, respectively. Thus, the commitment point probably ensures two and three successive S/M phases for T. socialis and G. pectorale to produce four- and eight-celled daughter colonies, respectively. These results suggested that changes in the threshold cell size for commitment to cell division may play an important role in the emergence and evolution of multicellularity in the volvocine algae.
C. reinhardtii 137c, T. socialis NIES-571, and G. pectorale 2014-0520-F1-1 were used in all experiments. Cultures were first grown in an inorganic (photoautotrophic) Standard Volvox medium (SVM) in 100 mL test tubes (approximately 3 cm in diameter; containing 50 mL culture) in continuous light of 100 µmol m−2 s−1 and aeration with 0.3 L min−1 at 20 °C for 3 d. On the third day, 1 µl of each culture was placed on top of SVM agar (1.0% agar; approximately 2 mm thick) in separate wells of a 24-well plate. Another thin layer of agar (0.8% agar; approximately 1 mm thick) was prepared and used to cover the cultures (T. socialis and G. pectorale only) to prevent drying. The cultures were observed using a light microscope and pictures were taken before transferring to dark conditions. After being kept in the dark for 24 h and 48 h at 20 °C, the cells were observed again and pictures were taken of the same areas. From the pictures, cells that divided were identified and the number of daughter cells produced was counted. The pictures at 0 h (before dark incubation) were also used to measure and calculate cell volumes based on the formula [4/3π(l/2)(w/2)2] (“l” and “w” indicate length or longitudinal distance and width or latitudinal distance, respectively).
Synchronous culture.T. socialis cells were precultured in SVM medium in 700 mL flat bottles (approximately 5 cm in depth; containing 500 mL culture) in the dark and aeration with 3.0 L min−1 at 20 °C for more than 3 h and then synchronized by subjecting the culture to a 12 h light (130 µmol m−2 s−1) and 12 h dark cycle. The same culture conditions were applied for synchronizing G. pectorale cells, except that the temperature was 25 °C.
Percentage determination of dividing cells and committed cells.During the third round of the light/dark cycle, cells were sampled every 2 h during the light period and every 4 h during the dark period. For each sample, the numbers of dividing and non-dividing cells were counted with the use of a light microscope, and the percentage of dividing cells was calculated. To determine the percentage of committed cells, the culture samples (T. socialis was only sampled during the light period) were placed onto agar, covered with another layer of agar, as described above, and then incubated in the dark for 24 h. The percentage of cells that divided after the dark incubation was counted.
Immunoblotting analysis to determine the expression level of an S phase marker.Another 10–15 mL of cells was sampled during the third round of the light/dark cycle for immunoblotting analysis. The samples were centrifuged to remove the culture medium and the cells were resuspended in SDS-PAGE sample buffer (50 mM Tris, pH 6.8, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol, and 0.01% bromophenol blue). The samples were kept at −80 °C until required for further steps. The protein concentration in each sample was determined using an XL-Bradford protein assay kit (APRO Science, Japan). An equal amount of protein from each sample was loaded into each well of the gel and separated by SDS-PAGE. The proteins were then transferred from the gels to polyvinylidene difluoride membranes and blocked with 5% skim milk dissolved in Tris-buffered saline (TBS) supplemented with 0.2% Tween-20 (TTBS) at room temperature. The membranes were then incubated with an anti-C. reinhardtii FtsZ1 antibody20) (at a dilution of 1:4,000) for 30 min, washed with TTBS three times (10 min each), and then incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody diluted 1:25,000. The membranes were washed with TTBS three times and signals were detected using an ECL Plus Western Blotting Detection Reagents (GE Healthcare) and ImageQuant LAS400 chemiluminescent detection system (GE Healthcare).
To determine the number of successive cell divisions that occurred after the commitment point, commitment assays were performed for C. reinhardtii, T. socialis, and G. pectorale (Fig. 1). Cells of respective species were cultured in continuous light (100 µmol m−2 s−1) with aeration with ambient air at 20 °C in a photoautotrophic (inorganic) medium. Then, logarithmically proliferating cells were placed on agar plates containing medium and incubated at 20 °C in continuous dark to stop further cellular growth. It should be noted that there were two layers of agar that sandwiched the cells to prevent them from drying, especially for G. pectorale cells, which showed signs of drying without the top agar, and could not complete cell division properly. This observation was consistent with the previous report that G. pectorale cells did not grow on agar plates.21) In this commitment assay, cells were expected to complete cell division if they had grown beyond the threshold size for cell division and passed the commitment point before they were incubated on plates in the dark. In our culturing conditions, cells finished cell division, whenever it occurred, in 24 h on plates in the dark and we did not observe any further cell division events from 24 h to 48 h after the onset of dark incubation (Fig. 1A–F). Thus, we examined the number of daughter cells that had been produced at 24 h after dark incubation.
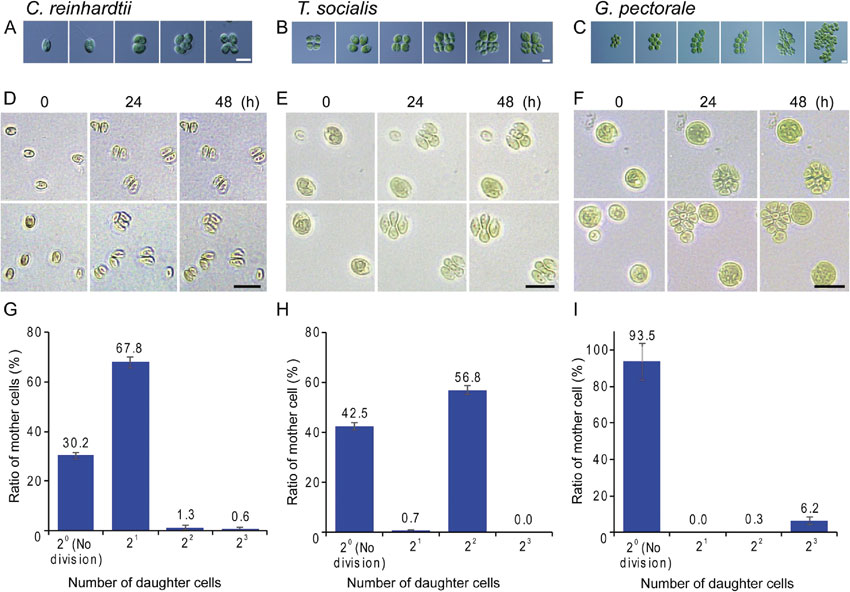
Number of successive cell divisions in C. reinhardtii, T. socialis, and G. pectorale. Microscopic images of cells showing processes in which mother cells grow and then divide, producing a defined number of daughter cells: two or four daughter cells in C. reinhardtii (A), four daughter cells in T. socialis (B), and eight daughter cells in G. pectorale (C). To determine the number of successive cell divisions, log phase cells cultured in an inorganic medium in continuous light asynchronously were plated on agar plates containing medium and incubated in the dark to stop cellular growth while allowing committed cells to divide. The images of the cells just before (0 h) and at 24 h and 48 h after the dark incubation on plates are shown for C. reinhardtii (D), T. socialis (E), and G. pectorale (F). The number of daughter cells produced (number of cell divisions) was counted 24 h after the onset of the dark incubation on plates. The results for C. reinhardtii (G), T. socialis (H), and G. pectorale (I) are shown. The error bar indicates the standard deviation of three biological replicates. Scale bar = 10 µm (A, B, C) and 20 µm (D, E, F).
In our culturing conditions, approximately 70% C. reinhardtii cells divided one, two, or three times on plates producing two to eight daughter cells. The major fraction was two daughter cells produced by one division. Two or three successive divisions occurred in smaller proportions (Fig. 1A, D, G). In contrast, approximately 60% T. socialis cells divided on plates in the dark, and most of the cells underwent two successive cell divisions producing four daughter cells (Fig. 1B, E, H). Regarding G. pectorale, approximately 6% of the cells divided on plates in the dark, and most of the cells underwent three successive cell divisions producing eight daughter cells (Fig. 1C, F, I). These results suggested that the number of cell divisions was limited to two or three in T. socialis and G. pectorale, thus always producing a four- or eight-celled daughter colony from a single mother cell, respectively. This was in contrast to C. reinhardtii that produced unicellular daughter cells.
Cell size at which commitment to cell division occurred in C. reinhardtii, T. socialis, and G. pectorale.In order to determine the minimal cell size beyond which cells can complete their cell cycle, cell volumes of all the three species before dark incubation on plates were determined and the data were integrated into the results of the number of cell divisions (Fig. 2). Cell length and width was measured and the cell volume was calculated based on the cellular ellipsoidal shape. We also determined the volume of daughter cells produced by cell division after 24 h dark incubation.
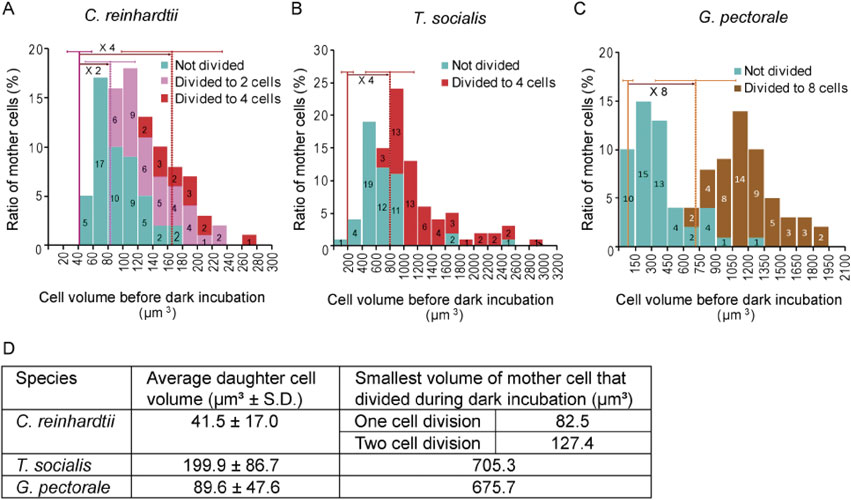
Relationship between cell size and the number of successive cell divisions in C. reinhardtii, T. socialis, and G. pectorale. The experimental conditions were the same as in Fig. 1. Cell volumes before dark incubation on plates were determined (n = 100 for each) and are presented in the histogram. Different colored bars differentiate cells that did not divide, divided once producing two daughter cells, divided twice producing four daughter cells, and divided three times producing eight daughter cells. On each histogram, the average size of daughter cells after cell division is also shown as a straight vertical line with the standard deviation, and the dotted line indicates the expected two-, four-, or eight-fold of the daughter cell size (A–C). The average size of daughter cells after cell division and the smallest size of mother cells that divided during dark incubation are also summarized in the table (D).
The results showed that T. socialis, which always undergoes two successive cell divisions to produce four daughter cells, whenever cell division occurs, had a commitment size (the smallest size of cells that underwent cell division on plates in the dark) that was close to four-fold of the average daughter cell volume (Fig. 2B). Regarding G. pectorale, which undergoes three successive cell divisions to produce eight daughter cells, the commitment size was nearly equal to eight-fold the average daughter cell volume (Fig. 2C). In contrast to these two species, the commitment size of C. reinhardtii cells were roughly equal to two-fold the average daughter cell volume, as shown previously9),10) (Fig. 2A). In addition, some of the cells that had grown beyond four-fold of the average daughter cell volume before dark incubation underwent two successive cell divisions producing four daughter cells in the dark, as shown previously9),10) (Fig. 2A).
These results indicated the correlation between the minimum number of successive cell divisions and the commitment cell size: two times the average newly born daughter cell volume for unicellular C. reinhardtii, four times for four-celled T. socialis, and eight times for eight-celled G. pectorale.
Position of the commitment point in T. socialis and G. pectorale in their cell cycle.Previous studies showed that the commitment point, at which a decision to divide or not is made, lies shortly before G1/S transition in C. reinhardtii.7),8),10),12),14),22) In order to examine whether this was also the case for T. socialis and G. pectorale, respective cells were synchronized by subjecting them to 12 h light/12 h dark cycle. During the third round of the light/dark cycle, temporal changes in the percentage of committed cells (using the commitment assay described above), level of an S phase marker protein in the culture (using immunoblotting), and percentage of cells in which daughter cells were formed, were examined (Fig. 3). As an S phase protein marker, the chloroplast division protein FtsZ1 was chosen because a previous study showed that it was expressed specifically during the S phase in eukaryotic algae including C. reinhardtii.20) In addition, the amino acid sequence of FtsZ1 is relatively highly conserved in green algae and the antibody against C. reinhardtii was prepared in our previous study.20)
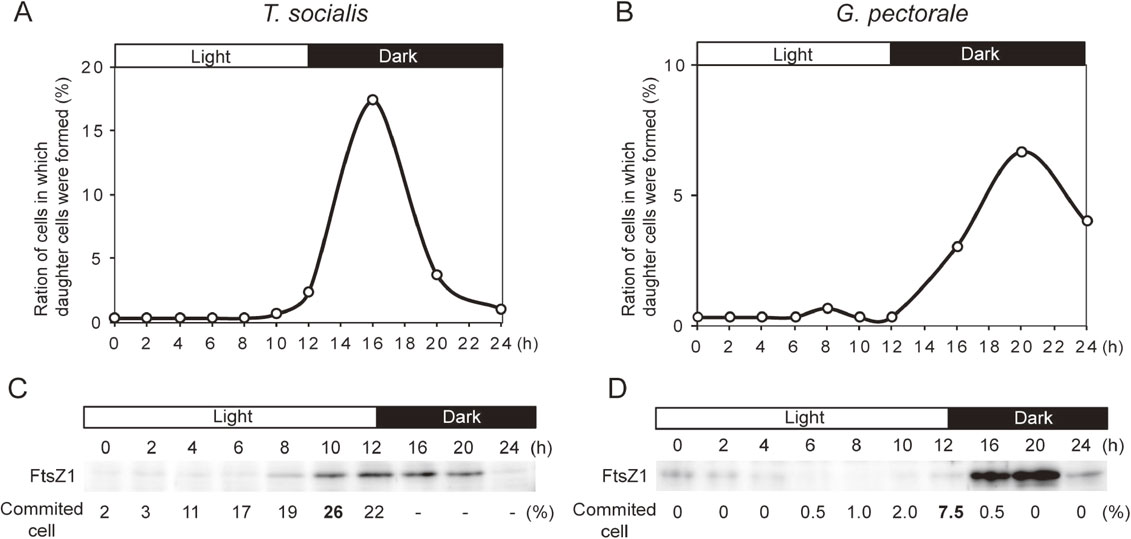
Changes in the percentage of committed cells, level of an S phase marker protein FtsZ1, and percentage of cells in which daughter cells were formed in T. socialis and G. pectorale. T. socialis, and G. pectorale were synchronized by a 12 h light/12 h dark cycle. The onset of the third round of the light/dark cycle is defined as hour 0. The percentage of cells in which daughter cells were formed are shown for T. socialis (A) and G. pectorale (B). The S phase protein marker, FtsZ1, was detected by the immunoblotting analyses for T. socialis (C) and G. pectorale (D). The percentage of committed cells before cell division are indicated for T. socialis (C) and G. pectorale (D).
In the synchronous culture of T. socialis, the committed cells before cell division peaked at 10 h (26%; Fig. 3C), FtsZ1 levels peaked at 12–16 h (Fig. 3C), and the cells, in which daughter cells were formed, peaked at 16 h (Fig. 3A). In the synchronous culture of G. pectorale, the committed cells before cell division peaked at 12 h (7.5%; Fig. 3D), FtsZ1 level peaked at 16–20 h (Fig. 3D), and the cells in which daughter cells were formed peaked at 20 h (Fig. 3B). In the commitment assay using cells cultured synchronously in a light/dark cycle (Fig. 3), the number of daughter cells were mostly four for T. socialis and eight for G. pectorale, which was consistent with the results obtained using asynchronous culture (Fig. 1). Thus, both T. socialis and G. pectorale cells are committed before S phase, as shown in C. reinhardtii. It was suggested that cell size is monitored in the late G1 phase to decide whether or not to enter into the S phase in these species.
Tetrabaena and Gonium evolved from a Chlamydomonas-like unicellular ancestor earlier than other multicellular volvocine algae.17) Cells of both species shared a high similarity with C. reinhardtii and they proliferate by a multiple fission cell cycle. Here, we have shown that T. socialis and G. pectorale cells passed the commitment point only when they had grown at least four- and eight-fold that of the original new born daughter cell size, respectively, in contrast to C. reinhardtii, which is committed when it has grown only beyond two-fold. These differences in threshold cell size for commitment may be the basis of multicellularity observed in T. socialis and G. pectorale, which ensure that the cells invariably undergo two (T. socialis) and three (G. pectorale) successive cell divisions once committed to produce four- and eight-celled colonies, respectively, as observed in this study (Fig. 4).
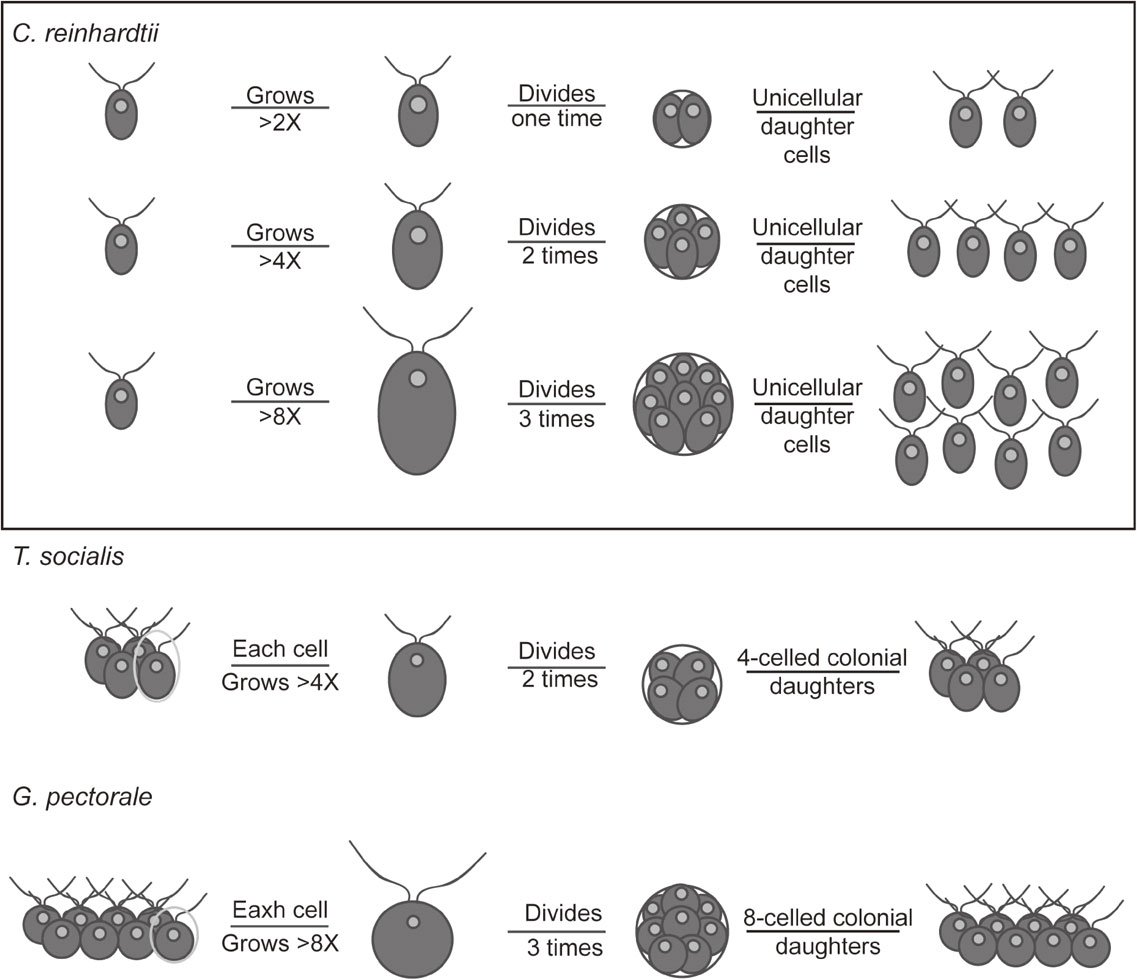
Schematic diagram of the progression of cell division depending on cell size in C. reinhardtii, T. socialis, and G. pectorale. In order to perform cell division, C. reinhardtii, T. socialis, and G. pectorale have to grow at least two-, four-, and eight-fold, respectively, during G1 phase. Depending on the mother cell size, C. reinhardtii undergoes two or more rounds of cell division, thus ensuring a relatively uniform-sized population of daughter cells. For T. socialis and G. pectorale, the cell division illustrated is only for one cell in the mother colonies.
Previous observations showed that the cells of Pandorina spp., which consist of eight to 32 cells grow many fold and then undergo several rounds of cell division.23),24) In a similar manner, the asexual reproductive cells (gonidia) of Volvox carteri undergo a rapid series of 11–12 cell division events with a marginal cell growth.24),25) Thus, changes in the threshold cell size for commitment was most likely involved also in evolution of other members of volvocine algae.
In C. reinhardtii, recent studies suggested that the RB-E2F-DP pathway, which is conserved in eukaryotes, is involved in size control at the commitment and subsequent G1/S transition in C. reinhardtii.12),15),16) RB represses the G1/S transition by binding to the E2F-DP heterodimer, which in turn binds certain specific cis elements of S phase genes.26)–28) In animal cells, G1 cyclins such as cyclin D accumulate during the G1 phase as a result of cell growth, and CDK-G1 cyclin phosphorylates RB.26)–28) This phosphorylation dissociates RB from E2F-DP, which then activates the transcription of S phase genes.26)–28) In C. reinhardtii, mutations in the RB homolog MAT3 resulted in a decreased commitment size and this phenotype was rescued by a mutation in DP or E2F.12),16)
Regarding the evolution of cell cycle control in volvocine algae, a recent comparative genomic study in C. reinhardtii, G. pectorale, and Volvox carteri showed that cyclin D genes displayed elevated dN/dS ratios, a signature of adaptive evolution, compared with other cell cycle regulators. In addition, the primary structure of the RB protein exhibited a considerable variation among the three species, although the actual functional differences remain uncertain at present.29) When C. reinhardtii RB was replaced with G. pectorale RB in C. reinhardtii, the genetically modified C. reinhardtii exhibited a colonial morphology that was composed of two to 16 cells.29) Given the results of this comparative genomic study and our observations here, it was suggested that evolutionary changes in G1 cyclin and the RB-E2F-DP pathway led to a change in the threshold cell size for commitment for cell division. Theoretically, if more G1 cyclin (cyclin D) accumulation by cellular growth is required to phosphorylate and inactivate RB by evolutionary changes, the threshold cell size will increase. For example, when the affinity between cyclin D and RB is reduced by evolutionary changes in these proteins, an increase in the level of cyclin D associated with cellular growth for the inactivation of RB and thus an increase in the threshold cell size for cell division will occur. In this regard, further studies that relate evolutionary changes in G1/S regulators and cell size for commitment will yield further mechanistic insights into the evolution of the multicellularity.
C. reinhardtii cells produce variable numbers of daughter cells: two, four, eight (as observed in this study), 16, or 32 (observed in previous studies9),10),30)). This variation is probably explained by the combination of commitment by at least a two-fold growth and circadian gating of S/M phase only during the (subjective) night. Even in an asynchronous culture in continuous light, the activity of each cell is under the regulation by its own circadian clock. Even when a cell has passed commitment by two-fold growth, the cell does not divide immediately. The cell grows further until a subjective night, when the cell is permitted to undergo the S/M cycle. In this case, there will be a variation in cell size among post-committed cells. During the (subjective) night, larger cells undergo two or more rounds of S/M phases depending on their cell size to produce a relatively uniform-sized population of daughter cells.
In contrast to C. reinhardtii, in this study, we observed only two successive cell divisions in T. socialis (Figs. 1 and 2), even though a certain population had grown by more than eight-fold before cell division (Fig. 2). In a similar manner, in the G. pectorale strain that was used in this study, four-celled (observed in our culturing condition) or eight-celled colonies have been observed29) but 32 (or more)-celled colonies have never been observed. Thus, the third and fifth cell divisions are inhibited in T. socialis and G. pectorale, respectively. However, it is theoretically likely that, for example in T. socialis, a cell undergoes four successive cell divisions to produce four four-celled colonies (16 cells in total), if cellular growth during subjective daytime can be accelerated by changing the cultivating condition.
Regarding the mechanism that determines the number of successive cell divisions depending on cellular size, a recent study in C. reinhardtii suggested that the “sizer” protein CDKG1 accumulated in accordance with cellular growth and was then consumed in a stepwise manner by successive cell divisions until the level became insufficient for a further round of cell division. Given this information on C. reinhardtii, the threshold CDKG1 level for a further round of cell division also probably changed during the evolution of four-celled Tetrabaena and eight- or 16-celled Gonium from unicellular Chlamydomonas-like ancestors. A recent study also suggested that CDKG1 acts though the RB and G1-cyclin (cyclin D) pathway, evolutionary changes in G1 cyclin and RB, as discussed above, were also likely the cause of a change in the control of the number of successive cell divisions besides that in cellular size for commitment.
Other than volvocine algae, many other algae with a coenobium structure proliferate by a defined number of divisions in a multiple fission cell cycle. In addition, some other unicellular algae, such as cyanidialean red algae, produce at least four or eight daughter cells in mother cells before hatching out of the mother cell wall and the minimum number of cell divisions varies depending on the strain.31) Thus, characterization of the mechanisms that restrict the minimum number of multiple fissions in other lineages will also be important to understand the evolution of a multiple fission cell cycle in eukaryotes.
We would like to thank Ms. Arakaki (the University of Tokyo) for information regarding the culture conditions for T. socialis NIES 571 and G. pectorale 2014-520-F1-1. This study was supported by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research 17H01446 (to S.-y.M.).