2017 Volume 93 Issue 2 Pages 51-63
2017 Volume 93 Issue 2 Pages 51-63
Single molecule detection has contributed to our understanding of the unique mechanisms of life. Unlike artificial man-made machines, biological molecular machines integrate thermal noises rather than avoid them. For example, single molecule detection has demonstrated that myosin motors undergo biased Brownian motion for stepwise movement and that single protein molecules spontaneously change their conformation, for switching to interactions with other proteins, in response to thermal fluctuation. Thus, molecular machines have flexibility and efficiency not seen in artificial machines.
Life is mysterious.
Owing to recent developments in biology, our understanding of life at the molecular level has rapidly progressed. In particular, essential proteins and biomolecules have been identified and their atomic structures have been determined. The structures and locations of these biomolecules inside a cell have been demonstrated by imaging technologies. Of special interest are the cellular functions regulated by molecular machines. For example, some molecular motors move along a protein track using ATP as an energy source, while others transduce signals within and between cells. These machines are sometimes understood in analogy to artificial machines. However, we know that molecular machines are fundamentally different from artificial man-made machines (Fig. 1). Our brain is, for example, flexible but not as fast or accurate as a computer. Computers, on the other hand, require a lot of energy. Human brains are estimated to use only 1–20 Watts based on brain temperature measurements1) while the Go-playing AI AlphaGo (Google DeepMind), which beat a world-class player Lee Se-dol, used 200,000 Watts. The challenge for biophysicists and bioengineers is to understand what accounts for this vast difference.

Molecular machines. Biological molecular machines, assemblies of biomolecules, play an important role in biological systems such as cells. The molecular machines (a) are compared with artificial man-made machines like a transistor (b). Major difference between two machines is thought to be the strategy against thermal noises (kBT ∼ 4 × 10−21 J). The input energy of a molecular machine was obtained as the chemical energy of ATP (20 kBT ∼ 8 × 10−20 J) and that of a transistor was obtained as (1/2) CV2 = 5,000 kBT (∼2 × 10−17 J), where C, capacity and V, voltage were assumed to be 50 aF and 0.9 volt, respectively (Personal communication, M. Taiji, Riken). The energy consumption of AlphaGo was obtained to be ∼200,000 Watts (J/sec) taking into account that it used 1,920 CPU (80 Watts/CPU) and 280 GPU (200 Watts/GPU) (https://en.wikipedia.org/wiki/AlphaGo).
In this review, we focus on the relationship between function and thermal noise as the reason for the difference. Artificial machines are designed to filter thermal noise because they are expected to disturb the function of these machines, resulting in less accuracy and efficiency. Thus, energy input (e.g., ∼10−17 J for a transistor) higher than thermal noise (∼4 × 10−21 J) is needed. This strategy allows artificial machines to achieve very accurate and high-speed performance. On the other hand, molecular machines are constituted of biomolecules, whose nanometer scale makes them extremely susceptible to thermal noise. The energy released from ATP hydrolysis, when used as the energy source for molecule machines, is ∼8 × 10−20 J, not high enough to avoid thermal noise (Fig. 1). Recently, single-molecule imaging and nano-detection techniques that we have developed, demonstrated that molecular machines and cells do not filter thermal noise, but rather use it to fulfill their functions. This may be the key to understanding the mechanisms of life.2)
We chose the myosin motor as a model molecular machine. Myosin is an actin-based motor that converts the chemical energy released from ATP hydrolysis into mechanical energy to generate muscle contraction and other types of cellular motility. One reason for choosing myosin is that it is the basis of muscle contraction, for which there exists extensive data at the macroscopic level. Furthermore, myosin shares many characteristic properties, such as enzymatic activities, energy transduction, molecular recognition, and self-assembly, with many other molecular machines, suggesting its properties can be extrapolated.
In spite of extensive actomyosin studies, the molecular mechanism of muscle contraction had remained controversial until single molecule detection techniques were available. Central events of myosin function include coupling the actomyosin interaction to ATP hydrolysis. Early molecular level studies investigated this coupling in the sliding assay system in which actin filaments move along myosin molecules attached to a glass slide,3),4) but because a variable number of myosin molecules interact with the actin at a given moment, conclusions at the single molecule level could not be made. We overcame this problem by developing new techniques to visualize fluorophore molecules and manipulate single protein molecules to measure the mechano-chemical coupling. This technique allowed us to monitor the sequence of events that result in ATP hydrolysis by the myosin molecule and the corresponding mechanical interactions between myosin and actin. It also allowed us to quantitatively measure thermal effects on coupling.
ATP and other biomolecules are too small to visualize with optical microscopy, but can be visualized with fluorescence microscopy. Because the fluorescence is detected at a different wavelength from the excitation light, only fluorescently marked molecules are monitored at the exclusion of other molecules, which means one can observe isolated myosin molecules by labeling them with a fluorescent probe.
High-resolution fluorescence microscopy was developed in the early 1990s. The key to this technique is the illumination of fields shorter than the wavelength of excitation light. This greatly reduces the background light and increases the signal-to-noise ratio. Using this method single fluorescent dye molecules were first observed in non-biological environments such as in solids and vacumes.5)–7) Following these findings, chemists and physicists tried to observe single fluorescent dyes in aqueous solution for biological use. These initial attempts were not successful because the fluorescence intensity is greatly decreased and instable due to collision of water molecules and ionized oxygen, and furthermore, the background noise due to Raman scattering by water molecules and dust is enormous. In 1995, we made the first observation of single fluorescent dyes in an aqueous solution.8) Here, we used total internal reflected fluorescence (TIRF) microscopy, in which an evanescent field created close to the glass surface (∼150 nm) by total internal reflection illuminates the dye and reduces the background light (Fig. 2(a)). This method requires extreme care of optics and solution preparation. This set-up, which allows us to monitor single fluorophore molecules labeled to biomolecules either bound to the glass surface or located close by, is advantageous for observing biological events for an extended time period until fluorophores are photo-bleached. For example, time-dependent images of molecular motors moving along molecular tracks (Fig. 2(b)),8) enzymatic reactions (Fig. 2(c)),9) protein-protein or protein-DNA interactions,10) ion channel in a lipid bi-layer11) and cell-signal processes on the surface of single cells12),13) have all been observed. Further improvements in single molecule imaging have been made with computer image analysis that tracks molecular position with nanometer and millisecond accuracy14),15) and with fluorescence spectroscopic techniques such as fluorescence resonance energy transfer (FRET)16),17) or orientation by polarization18),19) to determine the conformational changes of biomolecules. Confocal microscopy has been developed for single molecules free in solution.20)

Single molecule imaging. (a) Total internal reflection fluorescence (TIRF) microscopy. With this microscopy we visualized single fluorophore attached to biomolecules such as myosin and ATP in aqueous solutions. (b) Single molecule imaging of movement of myosin Vs along actin filaments. Myosin V is a molecular motor that transports vesicles in cells. Myosin Vs were labeled with GFP (green) and acting filaments were labeled with TM-Rhodamine (red). Bar, 2 µm. The fluorescent images look much bigger than the actual size of myosin molecules (∼20 nm). We can determine the center position of a fluorescent spot that is ∼1 µm in diameter with one nanometer accuracy by computer image analysis or a quadrant photodiode, and hence monitor the movement of a myosin with one nanometer accuracy. With this technique, we clarify the stepping mechanism of myosin V. (c) Single molecule imaging of the enzymatic cycles of ATP hydrolysis (ATP to ADP +Pi) by a myosin molecule. Fluorescent ATP analog, Cy3-ATP was used for fluorescently visualizing ATP. Combined with the single molecule manipulation technique this technique clarigy the mechano-chemical coupling of myosin (see Fig. 3).
One of the important strategies of single molecule measurements is to directly relate the input and output of molecular machines at the individual molecule level. Because many molecular machines use the chemical energy of ATP hydrolysis as an input, a method of monitoring the ATP hydrolysis reaction at the single molecule level was developed (Fig. 2(c)). Individual ATP hydrolysis cycles for a single myosin were measured using a fluorescent ATP analog, Cy3-ATP,21),22) which is hydrolyzed the same way as ATP in solution. When Cy3-ATP is associated with myosin fixed to a surface, a clear fluorescent spot appears at the position of the myosin molecule and disappears when the Cy3-ADP, the hydrolysis product of Cy3-ATP, is released. To assure that the observed Cy3-ATP and Cy3-ADP were associated with the same myosin molecule, the position of myosin was determined by labeling it with a different colored probe. The rate of association and dissociation averaged over many events for an individual myosin molecule is consistent with that obtained by conventional biochemical methods.23)
By hydrolyzing ATP, myosin is able to generate force, as measured by single-molecule nanomanipulation techniques. Single biomolecules can be captured by a glass needle24)–27) or by beads trapped by a laser.28)–30) The later traps particles 10 nm to 20 µm in diameter by the force of its radiation pressure, which is generated by using a high numerical aperture microscope objective to focus the laser light.31) The force myosin generates is in the piconewton range. The displacement of the glass needle or the bead in the optical trap is detected and converted into a force by calculation with the stiffness of 0.02–0.05 pN/nm.
To measure the mechanics of muscle myosin, the two ends of an actin filament are attached to optically trapped beads, and the actin filament is then brought into contact with a single myosin molecule fixed onto the surface of a glass pedestal. The myosin pulls on the actin with a force that is measured by the displacement of the bead, which can be measured with nanometer accuracy by projecting the bead onto a quadrant photodiode and measuring its differential output.
Simultaneous measurements of ATP hydrolysis and force by a single myosin molecule can be achieved by combining single molecule imaging with optical trapping nanometry to demonstrate the coupling between chemical and mechanical events (Fig. 3(a)).32) Interactions between myosin and actin change during the myosin displacement (upper trace in Fig. 3(b)) and with the fluorescence intensity of the Cy3-nucleotide (ATP or ADP) (lower trace in Fig. 3(b)).

Direct determination of mechano-chemical coupling of molecular motors. The molecular motors execute mechanical work using chemical energy. Single molecule detection demonstrates the mechano-chemical coupling by simultaneously measuring chemical reaction and mechanical events, which have been developed based on single molecule imaging and nano-manipulation, respectively (a). The results (b) and their interpretation (c) are drawn below. Myosin dissociates from actin filament (the displacement record drops to the original position) when (Cy3-)ATP binds to myosin (fluorescence increases) and myosin steps (displacement develops) when (Cy3-)ADP dissociates (fluorescence decreases), indicating the mechano-chemical coupling.
When an ATP molecule binds to the myosin head, the myosin head dissociates from the actin, the ATP fluorescence at the position of the myosin increases and at the same time displacement decreases to zero (from state (1) to state (2) in Fig. 3(b and c)). Then, ATP is hydrolyzed to ADP and Pi, which sequentially dissociate from the myosin and cause the myosin to rebind to the actin at a new location and generate displacement (force) (state (3) in Fig. 3(b and c)). Thus, each displacement corresponds to a single ATP hydrolysis reaction. The interval between steps and the duration of the myosin binding to actin filaments are consistent with the numbers obtained in bulk biochemical measurements.
Single molecule measurements have demonstrated that myosin steps are generated by the hydrolysis of a single ATP molecule, consistent with biochemical, physiological and structural experiments. These measurements have confirmed both weak and strong actomyosin binding states correspond directly to the nucleotide binding state of myosin and that the force generation step is coupled to the end product release step. However, only single molecule imaging could directly observe these relationships in sequence, while other macroscopic kinetic techniques such as stopped flow can only study one step from the sequence of events. Furthermore, these measurements revealed that the coupling of force generation and end product release was not always rigid but in some cases there was a delay between these steps where the energy was stored somewhere in the actomyosin system.32)
Myosin steps occur very rapidly, in the millisecond time range. Resolving the rising phase of the displacement would clarify the mechanism that generates myosin steps. We therefore have constructed a measurement system targeting this mechanism directly. This strategy requires a system with high stiffness. Because actin itself is insufficiently stiff it cannot be manipulated like the previously mentioned systems, and the system must target myosin directly.33)–35) In this technique, a single myosin molecule is captured directly to the tip of a cantilever that is connected to a stiff micro-glass needle (Fig. 4(a)). Single myosin molecules are attached at the cantilever’s biotinylated tail and the number of the molecules attached is measured using the photo-bleaching behavior of the fluorescence intensity.

Brownian steps of myosin during hydrolysis of a single ATP molecule. Single myosin molecules were captured to the tip of a cantilever attached to a glass-microneedle (a). Stepwize movement of myosin during hydrolysis of single ATP molecules (b, upper) was resolved into several 5.5 nm substeps (b, middle and bottom). Occasionally the substeps are backward (indicated by B) while majority of the substeps were forward. The myosin head steps on actin monomers on a protofilamentt during single ATP hydrolysis as sketched in (c) and the step movement are explained by biased Brownian movement under a potential generated by the interaction with actin filament (see text) (d).
With this method, we could monitor thermal noise in the probe’s displacement. The probe displacement fluctuated depending on the stiffness of the scanning probe and the actomyosin interaction (Fig. 4(b) upper panel). The probe position varied largely when myosin was in the ATP bound state, but much less so when myosin was in the strong binding state. The root-mean-square amplitude was 1.4–2.9 nm, which corresponds to a stiffness of 0.5–2 pN/nm. The maximum stiffness (∼2 pN/nm) is as large as that of the actomyosin crossbridge in muscle.36) Furthermore, the myosin head could consistently interact with actin without moving away from the actin filament because it was firmly affixed to a large scanning probe thus preventing diffusion. This slowed myosin motion, mimicking the condition in muscle.
This method revealed sub-steps within a single myosin step (Fig. 4(b) middle and bottom panels). The sub-steps occurred randomly, but had a constant size and were mostly directed forward but sometimes backward. The size of the sub-steps was determined by computing the histogram of the pairwise distances of all the data points of the rising phase of the displacement and found to be 5.5 nm. This size coincides with the interval between neighboring actin monomers subunits on one of two strands in an actin filament. Additionally, for a single ATP hydrolysis cycle there was a random distribution of the number of steps, ranging from one to five, i.e., the 5.5-nm steps were only loosely linked to the ATP hydrolysis cycle. The Gaussian nature of the motion and the length of the step are compelling indications that the myosin head walks or slides along the actin monomer, that is to say myosin transverses actin by Brownian motion34) (Fig. 4(c)). Importantly, the steps are most prevalent in an single direction, meaning it is not due to thermal diffusion alone but rather are biased forward. Similar biased Brownian motion was observed for a single headed kinesin family protein, KIF1A that transports vesicles along microtubules in cells.37)
The relationship between the force and the velocity was determined and plotted on a curve so as to allow a quantitative comparison of the mechanical properties of individual myosin motors with those of muscle. To cover a large range of forces, we acquired data with higher needle stiffness. The force-velocity curves of single myosin molecules were obtained using the step size and dwell time. Including the backward steps, the curves agreed precisely to previously established data in muscle.38)
The work done per 5.5-nm sub-step was estimated as the energy needed to pull a linear spring. The maximum work was 7.4 pN nm (= 7.4 × 10−21 J or 1.8 kBT) at high needle stiffness and the maximum work done during one ATP hydolysis cycle was 31 pN nm (= 7.2 kBT) as measured by taking the number of the sub-steps during a single ATPase cycle. Therefore, the highest efficiency achieved by individual myosin molecules when converting chemical energy into mechanical work is approximately 36% of the total energy released by one ATP hydrolysis cycle, approximately 20 kBT. This result matches highest efficiency found in contracting muscle fibers.39) The striking similarity of the plotted force-velocity relationship and chemical energy conversion efficiency seen in individual actomyosin molecules to muscles, the larger ensemble system to which the subunit belongs, implies that the overall physiologic properties of muscle are due almost entirely to the properties of individual actomyosin motors. That is to say, the major mechanical and thermodynamic properties of muscle are essentially the effect of the intrinsic properties of the subunit.
Biased Brownian steps can be described by Brownian particles in an asymmetric potential with different activation barriers for forward and backward steps (Fig. 4(d)). It is estimated from the frequency of the steps that the activation energy difference is 2–3 kBT at zero load. The relative number of backward steps increases when the load is increased and becomes equal to the number of forward steps at 2.5 pN. Several models have been proposed to define this asymmetric potential.40)–42) The geometric arrangement of the actin filament interaction with myosin molecules that are attached to a large scanning probe affects the shape of the potential. The actin filament has a two-stranded helical structure, called a protofilament, which contains 7 monomers and rotates 180° per half helical pitch. Thus, a myosin attached to a probe finds the actin monomers to which it binds are rotated along the helix, causing the orientation of the myosin head and actin binding sites to change depending on their relative position. The result is an asymmetric potential that follows the actin helical pitch. It is impossible for the myosin head to exceed half the helical pitch of actin because of constraints caused by the probe. For the same reason, the number of sub-steps is limited. The distribution of the number of sub-steps is due to the distribution of the initial binding position in the actin helical pitch.
The myosin moves along an acting filament by biased Brownian motion. The next question is how the myosin head senses the potential slope and stops the Brownian motion in the nearly best position. To answer this question, we designed an experiment as shown in Fig. 5(a).43) For this we used a different kind of myosin that transports vesicles in cells. This is because this myosin steadily moves long distances without dissociating from an actin filament and hence it is easier to measure its movements than those of muscle myosin.
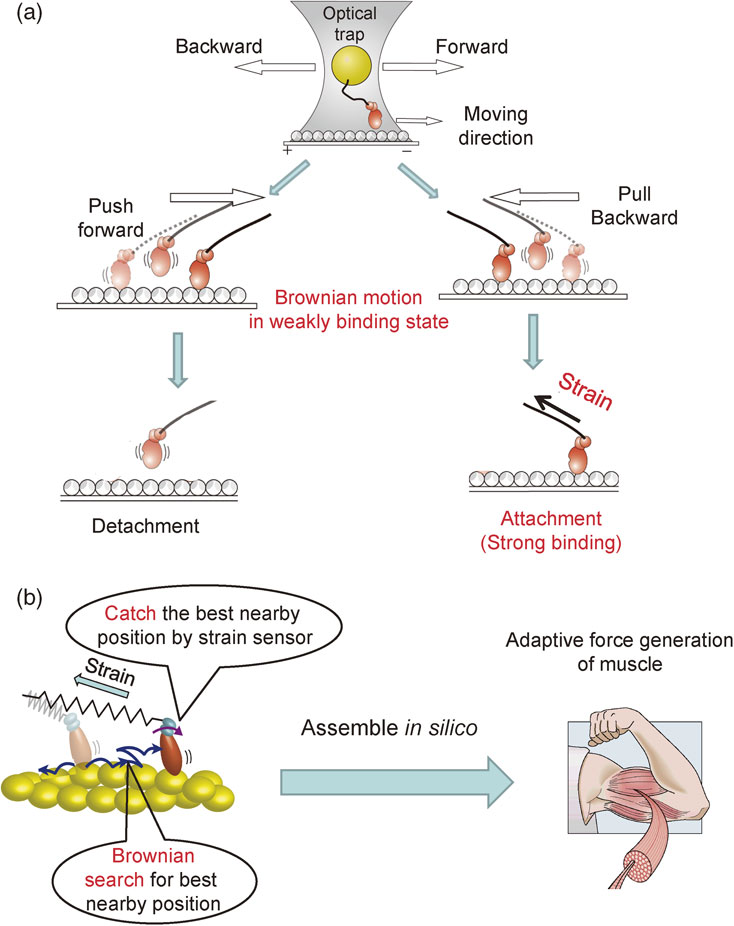
Brownian search and catch mechanism. Directional step of myosin is explained by strain-dependent preferential binding or transition from weak to strong binding of myosin to actin after Brownian search. The experimental evidence was obtained by single molecule measurements using optical trap, that is, the binding occurs in the presence of backward strain much more frequently than forward strain (a). Model for muscle based on single molecule measurements (b). Computer simulation demonstrates that the stochastic behavior of myosin molecules indicated from single molecule measurements explains the adaptive force generation of muscle.
Myosin heads undergo thermal diffusion randomly on the actin filament in the weak binding state in the presence of ATP where it attaches to and detaches from the actin filament rapidly in the µs time range. Eventually, the myosin head binds to the actin filament in the forward and backward directions, resulting in accelerated backward and forward strain, respectively. The mechanism of the transition from a weak-binding to a strong-binding state has been best explored in myosin.
In one experiment, a single-headed myosin molecule tethered to an optically-trapped polystyrene bead is moved back and forth rapidly in the µs time range along an actin filament by scanning the bead with an optical trap. When the myosin head in the weak-binding state is strained for a moment, it transitions to the strong binding or detachment. The strong bindings were seen, depending on the direction of the strain. Strong bindings were accelerated by 30 times when the myosin head was pulled backward, while detachments were accelerated by 5 times when the head was pushed forward (Fig. 5(a)). The results suggest that the myosin head has a strain sensor by which the myosin head senses the direction of movement and decides the position where the Brownian motion is stopped. Base on the results, we have proposed a Brownian search and catch model (Fig. 5(b)). The myosin head undergoes Brownian search for the best nearby binding site (attractor) and catches the best nearby binding site by the strain sensor, depending on the conditions.
In muscle, myosin and actin are assembled into a hierarchic structure in order to achieve contractions. To understand how the properties observed at the single molecule level apply to the macroscopic level, we built a simulation.
Our first stimulation demonstrated cooperative action among several myosin molecules when they undergo biased Brownian movement under asymmetric potential, which is depicted in Fig. 4(d). The distance a single myosin can move during the hydrolysis of a single ATP molecule is limited to half the helical pitch of the actin filaments, as described above. When multiple myosin heads undergo Brownian movement in the weak-bound state, the release of Pi from any one results in a rigor complex with actin. It has been demonstrated that the actin filament is rotated during sliding in vitro44)–46) and during force generation in muscle.47),48) In a biased Brownian model that includes multiple myosin heads, we assume that the actin filament is rotated 90° due to the formation of the rigor complex.34) An ATP molecule then binds to the rigor head to dissociate it from actin and the actin filament returns to its original orientation. The rotation of the actin filament changes the relative geometry between myosin and actin allowing the myosin heads to attach weakly and step. It was demonstrated that sliding distances per ATP increases to greater than 60 nm in muscle structure from 10–20 nm for isolated myosin, in agreement with experimental results using myosin filament and muscle fiber.49),50)
The Brownian motion of myosin and the search-and-catch mechanism evidenced from single molecule measurements are two key assumptions in the original Huxley model.51) By considering single molecule measurement results and muscle structure, we simulated the fundamental mechanical properties of muscle.52) The actomyosin interaction was reduced to two states: attached and detached. In the attached state, myosin follows a Brownian model and in the detached state it moves freely. The rate of attachment and detachment is assumed to depend on the direction of the movement, as in the original Huxley model.53)
The model with parameters obtained from single molecule measurements was quantitatively tested using two classical experiments for skeletal muscle: fast tension recovery after a small and fast increase in the isometric condition, and the velocity of contraction in the isotonic condition52) (Fig. 5(b)). A muscle fiber bearing a constant load and generating a tension T < T0 can contract at a constant velocity in a manner that depends on the load in a hyperbolic relationship. The simulated V/Vmax vs. T/T0 relationship fits very well with experimental data. Thus, our model, based on single molecule measurements, can predict the macroscopic properties of muscle using parameters derived from single molecule measurements rather than transition rate functions that are based on muscle fiber behavior in a phenomenological fashion.
Proteins are the main elements of molecular machines and play an important role in the machine function. Proteins themselves are sensitive to thermal fluctuations. To understand how individual protein molecules respond to thermal noise, we have studied dynamic changes of the conformational state of proteins using single molecule imaging. The combination of single molecule imaging with fluorescence spectroscopy techniques such as FRET allows us to monitor the conformational changes of proteins over time.
Actin filaments are the tracks for myosin movement and their conformation affects myosin motility. Single molecule FRET from specifically double labeled actin monomers within an actin filament have revealed that actin has multiple conformations that activate or inhibit the myosin motility53) (Fig. 6(a)). Moreover, there is a spontaneous conformational fluctuation between the two states even in the absence of myosin, however, the binding of actin to myosin drives the conformational population towards the active state and thereby, activates myosin motility.

Dynamic structure of protein molecules. Dynamic conformational changes of individual protein molecules are observed using single molecule fluorescence resonance energy transfer (FRET). The data demonstrated spontaneous changes of conformation among multiple conformational states. (a) Actin flucuates between an inactivated state and an activated state that accelerates myosin activity. The binding of myosin shifts the equilibrium to activated state. (b) Ras is among a refractory state and multiple conformational states suitable for interactions with different effectors such as RalGDS and Raf. The binding of one of the effectors shifts the equilibrium to conformational state specific for the effector.
The various conformational states of the protein molecule are in fact discrete thermodynamically stable areas within the topological free energy landscape. These, local minima are separated by energy barriers. The protein structures fluctuate when the energy barrier is on the same order of magnitude as thermal noise. The fluctuations can be explained by the amplitude of the local minima and the barriers between them, which vary with interactions between proteins and ligands and with environmental changes.
Actin’s spontaneous conformational fluctuations contribute to its function when it interacts with binding proteins. The binding of myosin causes actin conformational changes that enhance ATP hydrolysis activity of myosin, and initiates motility.54),55) These conformational changes are mediated along the actin filament, especially in the presence of the actin binding proteins tropomyosin and troponin. Thus, myosin activation is enhanced cooperatively and therefore effectively. This property is advantageous in the dynamic assembly of the actin filament and for actin function in live cells.
Another example of conformational fluctuations is in the signaling protein Ras. Ras conducts molecular switches to regulate signal transduction processes by interacting with various target proteins dependent on GTP in cells. How does this protein control multiple signals effectively? Single molecule FRET measurements have shown that Ras has multiple conformational states, a refractory state and multiple active states corresponding to various effectors56) (Fig. 6(b)). Even in the absence of effectors, Ras fluctuates among these multiple conformation states over time. The binding of effectors, such as Raf or RalGDS, decreases the population in the refractory state, as the effector preferentially binds to one of the activated states that exists before binding, and decreases the dynamic conformational fluctuations. Thus, its multiple conformations may allow Ras to interact with many target proteins to fulfill its multiple functions.57) An expanded understanding of how individual signaling molecules behave in live cells is imperative.
Single molecule detection has proven to be a powerful tool for the study of the unique working principles of biomolecular machines in comparison with artificial machines. Molecular machines of nanometer size are exposed to thermal noise and therefore thermal fluctuation. Our new technology has revealed that the molecular machines utilize the thermal fluctuation for function. This is contrasted by man-made machines, which filter these noises. The mechanisms utilizing thermal fluctuation for function are seen in various events at the level of biomolecules, molecular machines and cells. This suggests that the fluctuation mechanism is common in various life events58) (Fig. 7). Furthermore, a similar mechanism is suggested in visual perception processes. In our perception we experience spontaneous alterations between two possible percepts of Edwin Boring’s young/old woman (inside Fig. 7). This image can be interpreted two ways: a young woman or an old lady. When we observe this image, only one percept at any moment is perceived, however, the two percepts spontaneously alternate. The analysis of the interval time of the perceptional alteration suggests that perception processes can be described using similar equations used for molecular dynamics, even though perception depends on an extremely complicated neural network brain.59),60) Brain function imaging measurements will reveal the physical basis of these phenomenological stochastic processes in the near future.
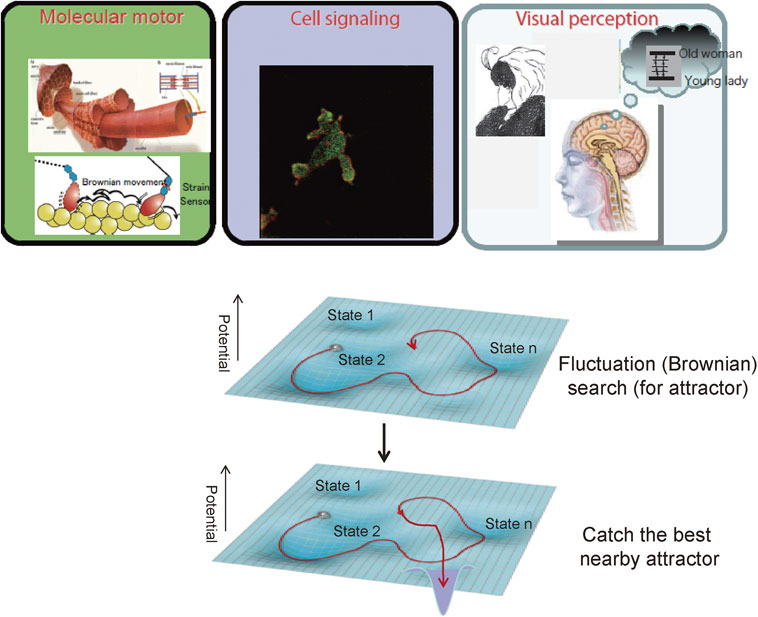
Common principle (fluctuation search and catch mechanism) works from molecules to the human brain. Fluctuation search and catch mechanism is seen in various biosystems in molecular machines, cells and brains, which are approached in completely different way. In this mechanism, a system undergoes fluctuation (Brownian motion) among multiple states (attractors) and catches the (nearby) best state (attractor) depending on the conditions. This mechanism makes biosystems highly autonomous and highly energy efficient which discriminates life from man-made machines.
All these results imply the possibility that stochastic mechanisms may play important roles in life events in the levels from molecules to individuals, making the mechanism of life different from man-made machines. Our world developed with traditional technology faces myriad problems related to environmental issues such as limited energy sources and global climate change. One way to overcome these problems is to develop new technologies based on the operation mechanisms of life. The fluctuation search and catch mechanism seen at various levels of life described in this review has the potential for engineering applications. Fluctuation search and catch mechanism have been applied to many areas of engineering such as robotics and communication networks.61) New communication networks, for example, could be built with a fluctuation search and catch mechanism, which works flexibly even in cases of accident or disaster. Machines and systems that use the fluctuation search and catch mechanism have been demonstrated to operate flexibly and with less energy consumption. The successful application of the fluctuation mechanism to engineering strengthens the idea of the importance of noise and benefits human society as well. Recently AI (artificial intelligence) technology has been demonstrated to be very powerful and greatly expected to bring out an innovation of engineering. However, a serious problem is that the present AI requires a huge amount of energy (Fig. 1). This problem should be overcome by learning the principle of life.

Toshio Yanagida was born in Hyogo prefecture in 1946. He graduated from Osaka University and received the Doctor of Engineering degree in 1976. He was a Professor at Osaka University’s Graduate School of Information Science and Technology from 1988, Department of Physiology and Biosignaling, Graduate School of Medicine from 1996, and the Graduate School of Frontier Biosciences from 2002. He opened the new frontiers for muscle contraction mechanism by developing new single molecule detection techniques as the leader of the Yanagida Biomotron Project, ERATO, JST in 1992–1997 and its successor, the Single Molecule Process Project, ICORP, JST in 1998–2002, where he has pioneered single molecule detection techniques. After these projects, he was Research Director of Soft Nano Machines and Research Supervisor of Novel Measuring and Analytical Technology Contributions to the Elucidation and Application of Life Phenomena, CREST, JST in 2004–2012. He has extended the idea of fluctuation mechanism from molecular motors to cells and brain. He is currently Director of both the RIKEN Quantitative Biology Center (QBiC) and of the Center for Information and Neural Networks (CiNet), NICT, while also holding the titles of Specially Appointed Professor, Osaka University, and Director, NEC Brain-Inspired Computing Research Alliance Laboratories. Finally, Dr. Yanagida has received a number of prestigious awards including the 25th Naito Memorial Science Promotion Award in 1994, the Imperial Prize and Japan Academy Prize in 1998, and an Asahi Award in 1999. He was also honored to be a Person of Cultural merit in 2013.
Yoshiharu Ishii was born in Tokyo in 1948. He graduated from Tokyo University of Education and graduate School of science, Nagoya University and received Ph.D. degree in biophysics. After he worked at department of muscle in Boston Biomedical Research Institute in 1983–1992, he joined the JST projects led by Prof. Toshio Yanagida as researcher and research manager. The projects include the Yanagida Biomotron Project, ERATO, JST in 1992–1997, the Single Molecule Process Project, ICORP, JST in 1998–2002, Soft Nano-machines Project in 2003–2008, and Novel Measuring and Analytical Technology Contributions to the Elucidation and Application of Life Phenomena, CREST, JST in 2008–2012. He currently works at the RIKEN Quantitative Biology Center (QBiC).